Studies in patients with spinal muscular
atrophy (SMA) show SPINRAZA
Achieved meaningful results across
age
groups—from infants to
adults1-3
Backed by the longest clinical trial
program to date in infants and
children.4 Supported by extensive
real-world evidence in
adults, teens,
and older children2,3,5-7
Click the tabs to see the data in the selected population
Pivotal trial: CHERISH1,8
The efficacy of SPINRAZA (12 mg) for later-onset SMA was demonstrated in CHERISH, a phase 3, sham-controlled pivotal trial. Study duration was 15 months and included 126 patients aged 2 to 9 years at screening.8
- Primary endpoint: least-squares mean change from baseline in motor function as measured by HFMSE at 15 months
- Results: SPINRAZA: 3.9-point change; Untreated: -1.0-point change
- The most common side effects were fever, vomiting, headache, and back pain
HFMSE, Hammersmith Functional Motor Scale—Expanded; SMA, spinal muscular atrophy.
Extensive real-world
evidence in SMA2,3,5-7
A growing body of real-world
evidence supports the use of
SPINRAZA in adults, teens, and
older children2,3,5-7
Biogen-sponsored pivotal trials for
SPINRAZA (12 mg) did not include adults
with SMA.
Click on the studies below for more.
| Publication | Study description | Population | Study duration |
|---|---|---|---|
|
Günther R, et al3
The Lancet Regional Health—Europe. 2024;39:100862 |
An independent, prospective, observational multicenter study in Austria, Germany, and Switzerland |
347 patients
|
Up to 38 months |
|
Łusakowska A, et al2
Orphanet Journal of Rare Diseases. 2023;18(1):230 |
A prospective, observational study at 2 centers in Poland (sponsored by Biogen) |
120 patients
|
Up to 30 months |
|
Hagenacker T, et al5
The Lancet Neurology. 2020;19(4):317-325 |
An independent, prospective, multicenter, observational cohort study |
139 patients
|
Up to 14 months |
Maggi L, et al6Journal of Neurology, Neurosurgery and Psychiatry. |
An independent, retrospective, multicenter, observational cohort study |
116 patients
|
Up to 14 months |
Coratti G, et al7Orphanet Journal of Rare Diseases. 2021;161(1):430 |
An independent, critical review and meta-analysis based on 19 peer-reviewed real-world data publications |
SMA Type 2 or 3 |
10 to 14 months follow-up |
SMA, spinal muscular atrophy.
Click the arrow for each study below to expand or close.
Günther et al The Lancet
Regional Health—Europe
Long-term outcomes with SPINRAZA: a real-world study of adults and teens across multiple measures of motor function for up to 38 months3
Study design: An independent, prospective, observational study that assessed 237 adult and teen patients with genetically-confirmed 5q-associated SMA who were treated with SPINRAZA (12 mg) for up to 38 months. Patients from the SMArtCARE registry were recruited between July 2017 and May 2022 in Germany, Switzerland, and Austria. Thirty-one patients were excluded due to insufficient follow-up and 11 discontinued SPINRAZA before completing the 14-month follow-up. Patients with missing data points were excluded from analysis.3

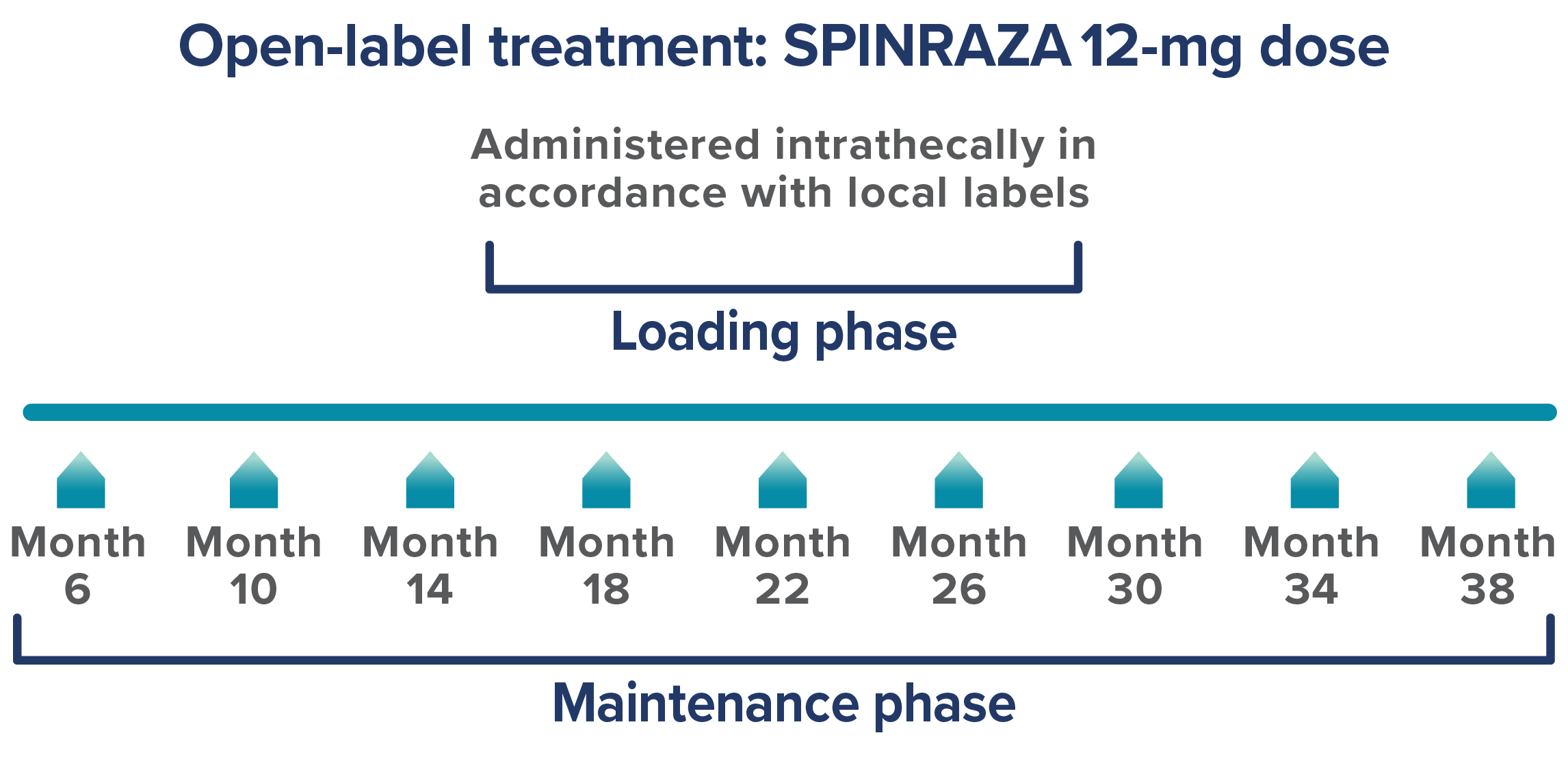
Please refer to the FDA-approved dosing schedule in the US Prescribing Information.
Study limitations3:
- Observational study without an untreated control group; this does not have the same class of evidence as a randomized, controlled trial
- Patient enrollment was limited to centers in Germany, Switzerland, and Austria. Treatment practices may vary by country
- Hammersmith Functional Motor Scale—Expanded (HFMSE) score interpretation is limited by floor effects in severely disabled patients (SMA type 2 and nonambulatory SMA type 3), which may have affected the results of the study
- Revised Upper Limb Module (RULM) score interpretation is limited by ceiling effects in less impaired patients (ambulatory SMA type 3), which may have affected the results of the study
- Motor assessments did not evaluate muscles required for swallowing or respiratory function
- Differences in loading dosing compared to approved SPINRAZA dosing schedule
Endpoints: Three functional outcomes were assessed at 14, 26, and 38 months of treatment and included 237, 171, and 120 patients, respectively3:
- HFMSE
- Primary endpoint: Mean change vs baseline
- Clinically meaningful improvement (defined as a change of ≥3 points)
- RULM
- Mean change vs baseline
- Clinically meaningful improvement (defined as a change of ≥2 points)
- 6-Minute Walk Test (6MWT) in ambulatory patients
- Mean change vs baseline
- Clinically meaningful improvement (increase in walking distance by ≥30 meters)
Safety: The safety was generally consistent with the known safety of SPINRAZA in clinical trials.1,3,8
- Safety was evaluated in 389 patients screened who had received at least one injection3
- 732 adverse drug reactions or procedure-related complications were documented in 91% of patients (n=353/389)
- 26% of patients (n=103/389) reported no adverse drug reactions; 64% of patients (n=250/389) reported at least 1 adverse reaction
- Most adverse reactions were post-lumbar puncture syndrome, headache, back pain, and infections
This registry was funded in part by Biogen.
6MWT, 6-minute walk test; FDA, Food and Drug Administration; HFMSE, Hammersmith Functional Motor Scale–Expanded; RULM, Revised Upper Limb Module; SMA, spinal muscular atrophy.
Long-term outcomes with SPINRAZA:
HFMSE, RULM, and 6MWT3
Improvement in mean HFMSE score vs baseline at each assessment3
Primary endpoint: Change in HFMSE score from baseline
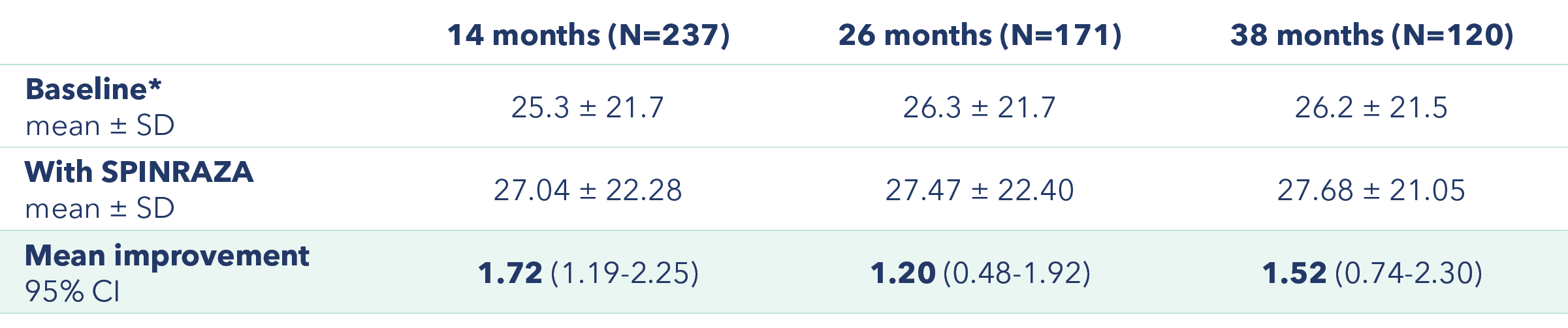
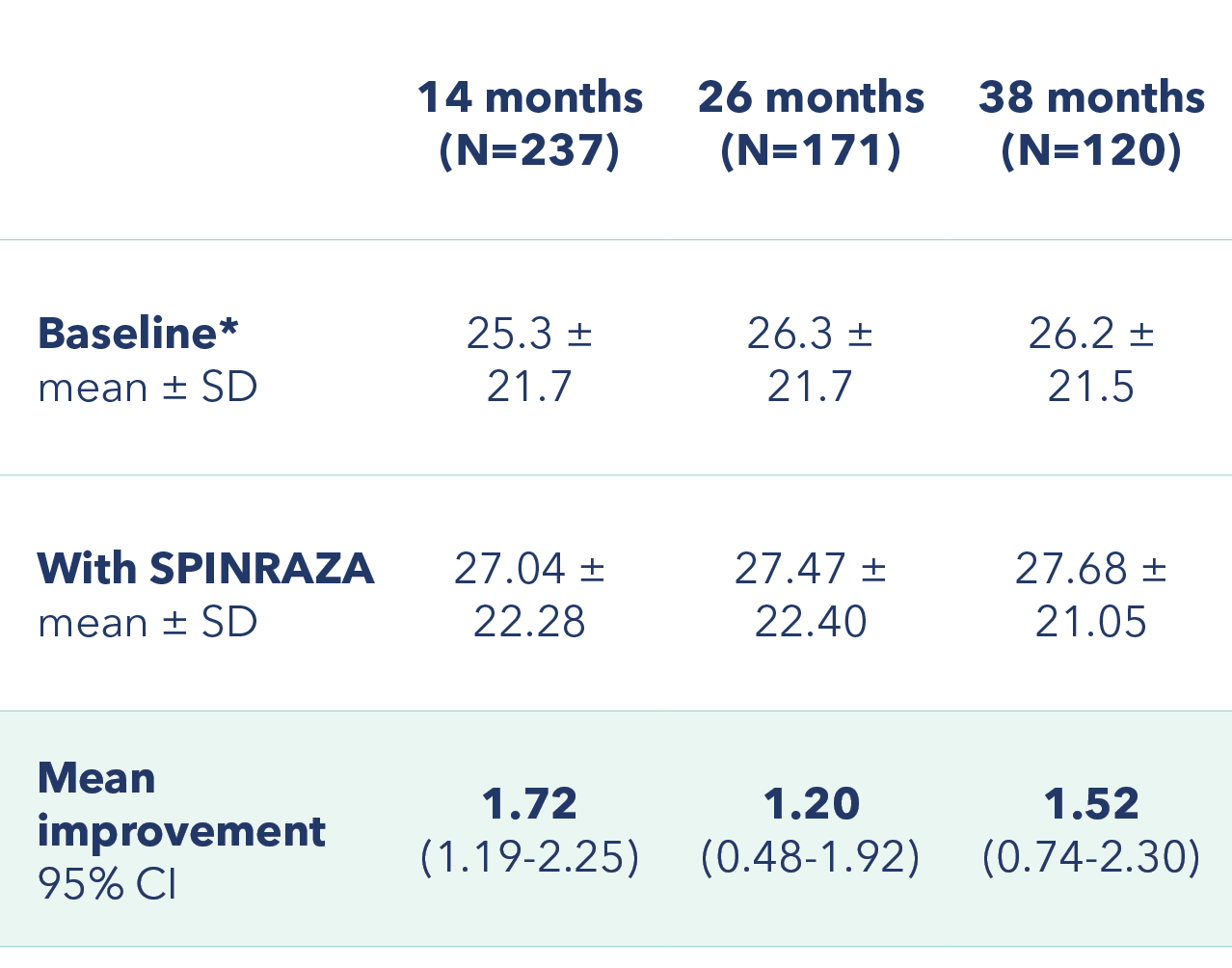
*At baseline 2 patients had a full score (66 points) and 55 patients scored 0 points.
Proportion of patients with clinically meaningful change in HFMSE score vs baseline up to 38 months
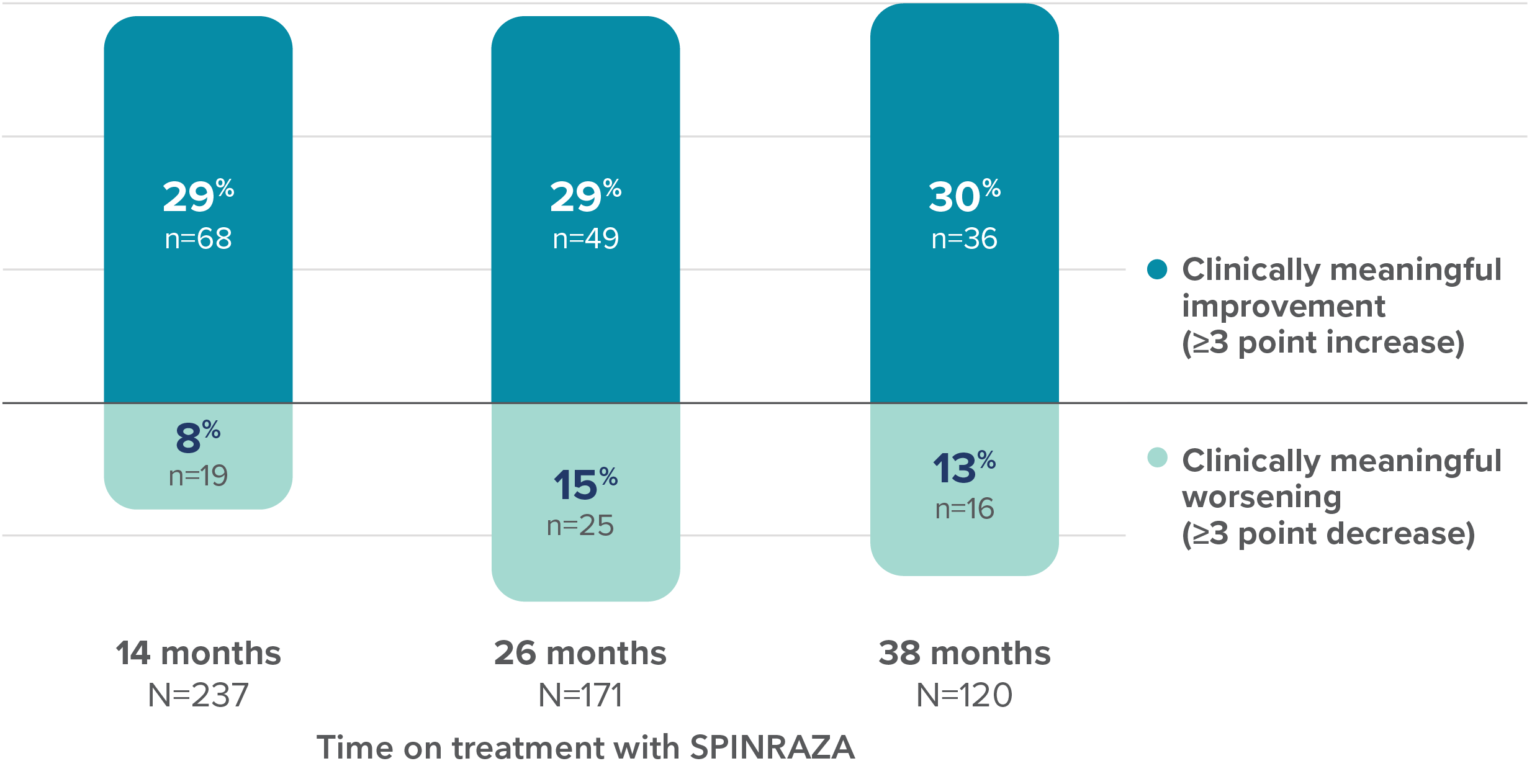
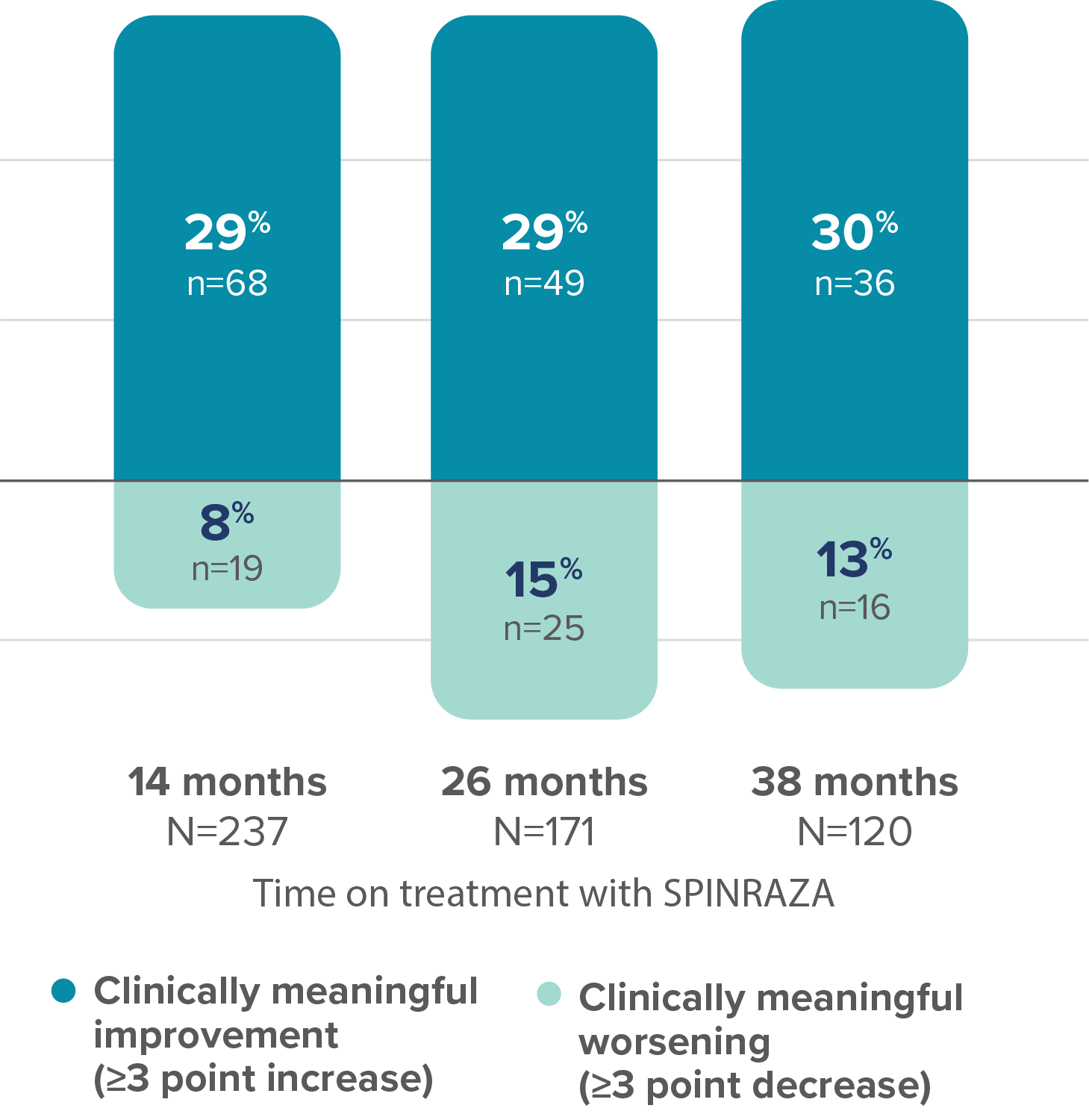
28 of the 68 patients with clinically meaningful improvement at 14 months maintained this improvement for ≥38 months3
- HFMSE interpretation may have been limited by floor effects; non-ambulatory patients could not perform some of the activities assessed by HFMSE, leading to lower achievable scores
6MWT, 6-minute walk test; Cl, confidence interval; HFMSE, Hammersmith Functional Motor Scale–Expanded; RULM, Revised Upper Limb Module; SD, standard deviation.
RULM scores improved from baseline at each assessment3
Mean improvement in RULM from baseline

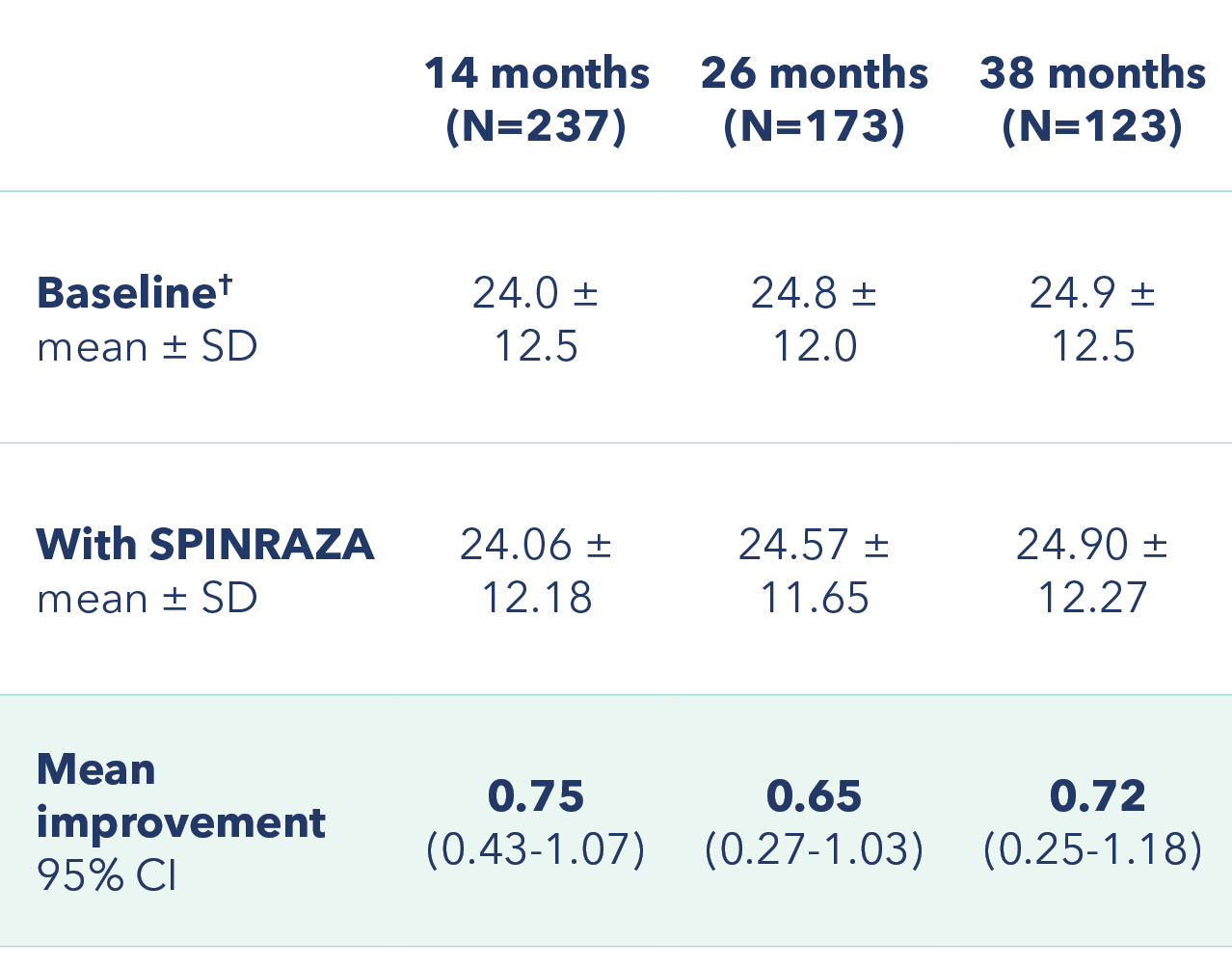
†At baseline 74 patients had a full score (37 points) and 17 patients scored 0 points.
Proportion of patients with clinically meaningful change in RULM score vs baseline up to 38 months
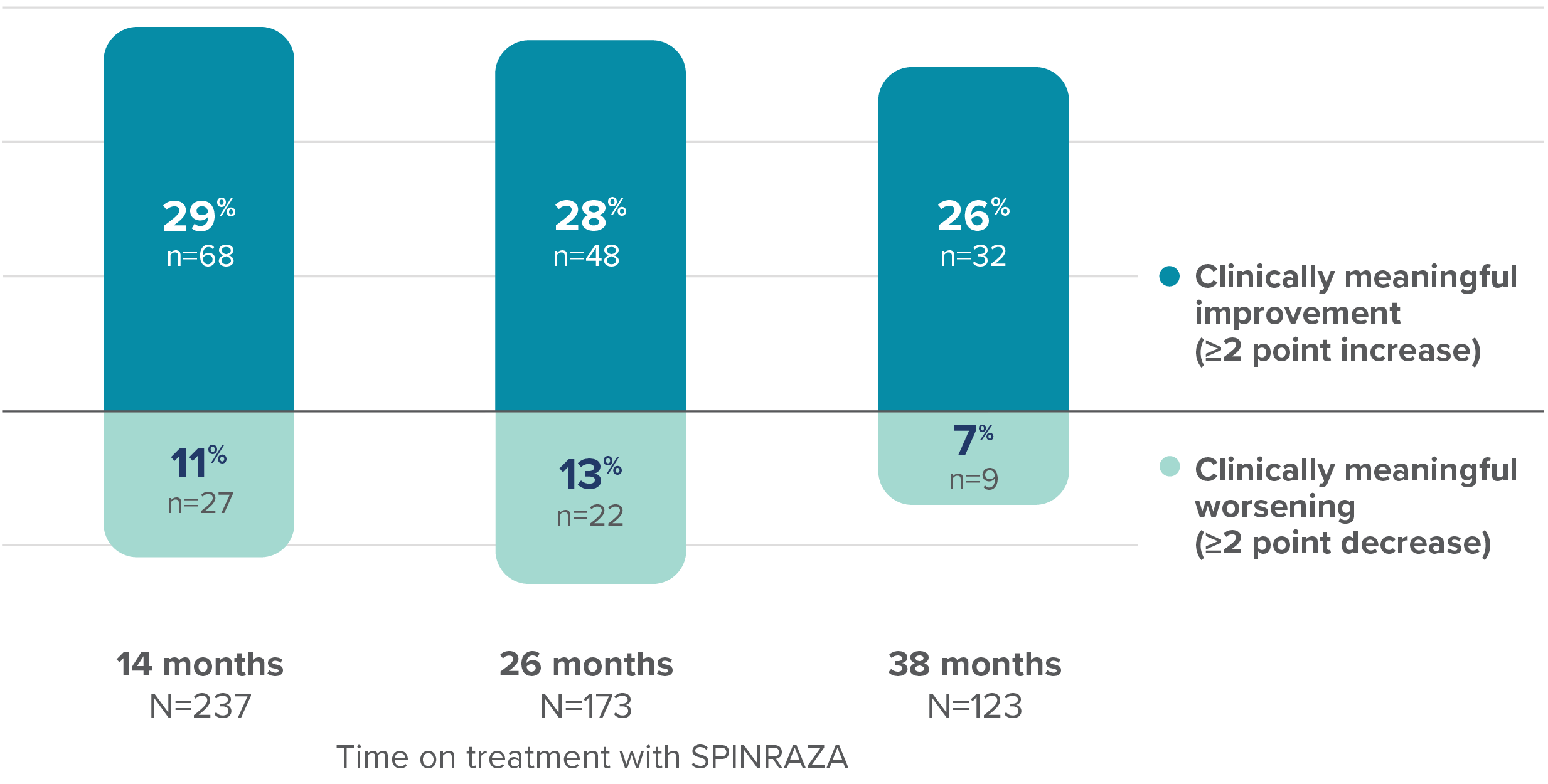
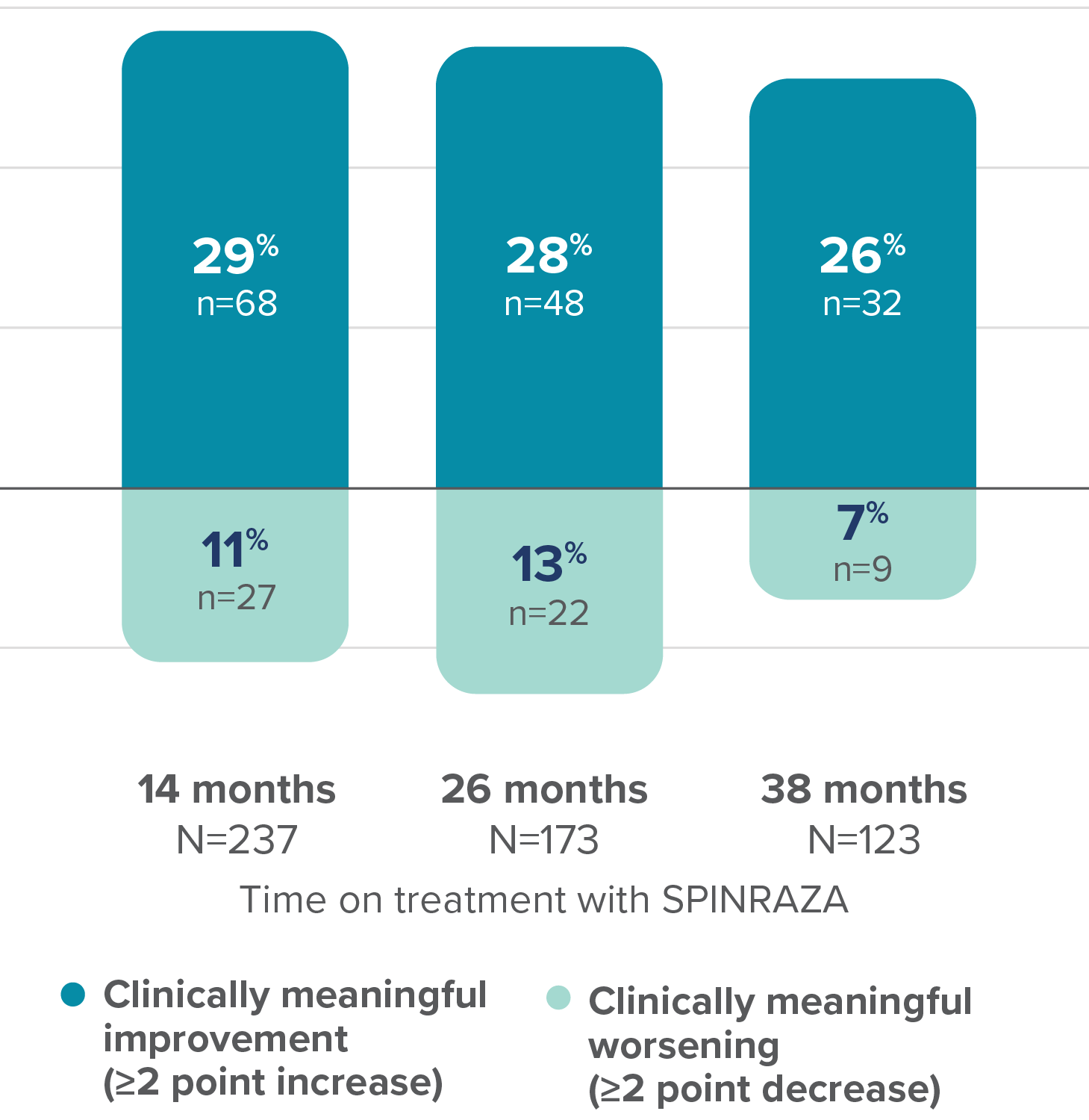
37% of patients (n=25/68) with clinically meaningful increase in RULM score at 14 months maintained this improvement at 38 months3
- RULM interpretation in ambulatory patients may have been limited by ceiling effects, which may have affected the results of the study
Cl, confidence interval; RULM, Revised Upper Limb Module; SD, standard deviation.
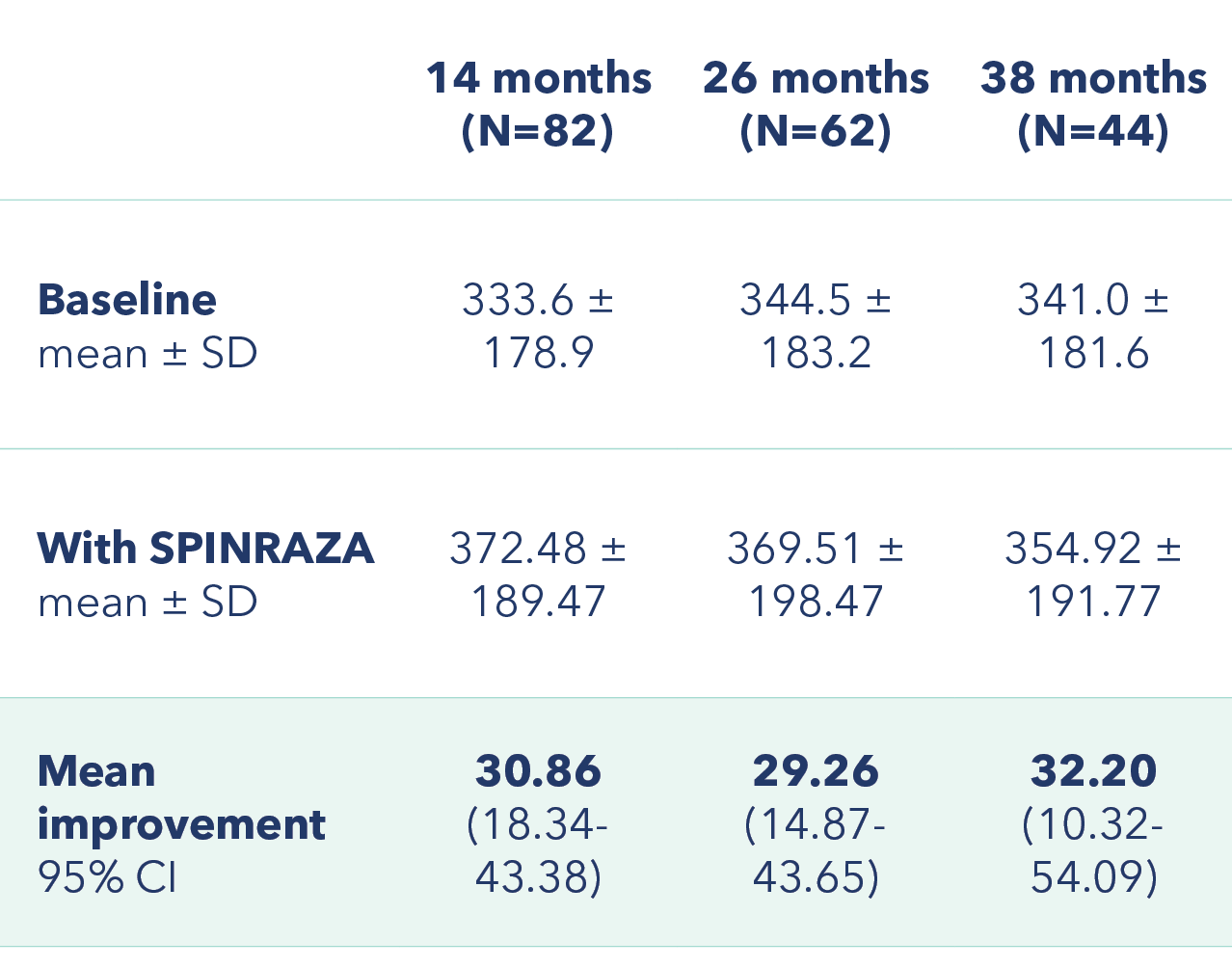
Improvement in mean walking distance from baseline in the 6MWT at each assessment in ambulatory patients3
Mean improvement in in walking distance (in meters) from baseline

Proportion of patients with clinically meaningful change in 6MWT score vs baseline up to 38 months
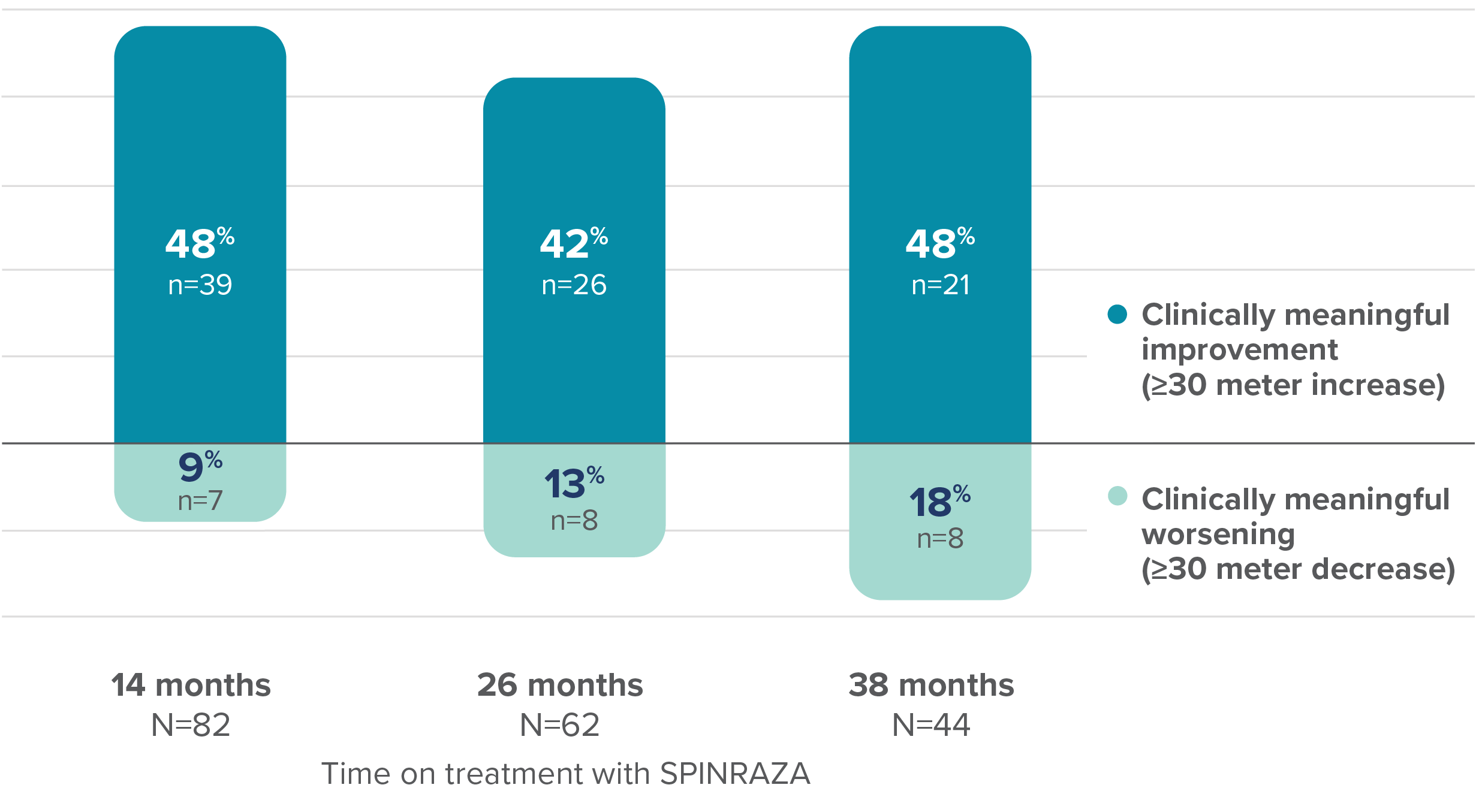
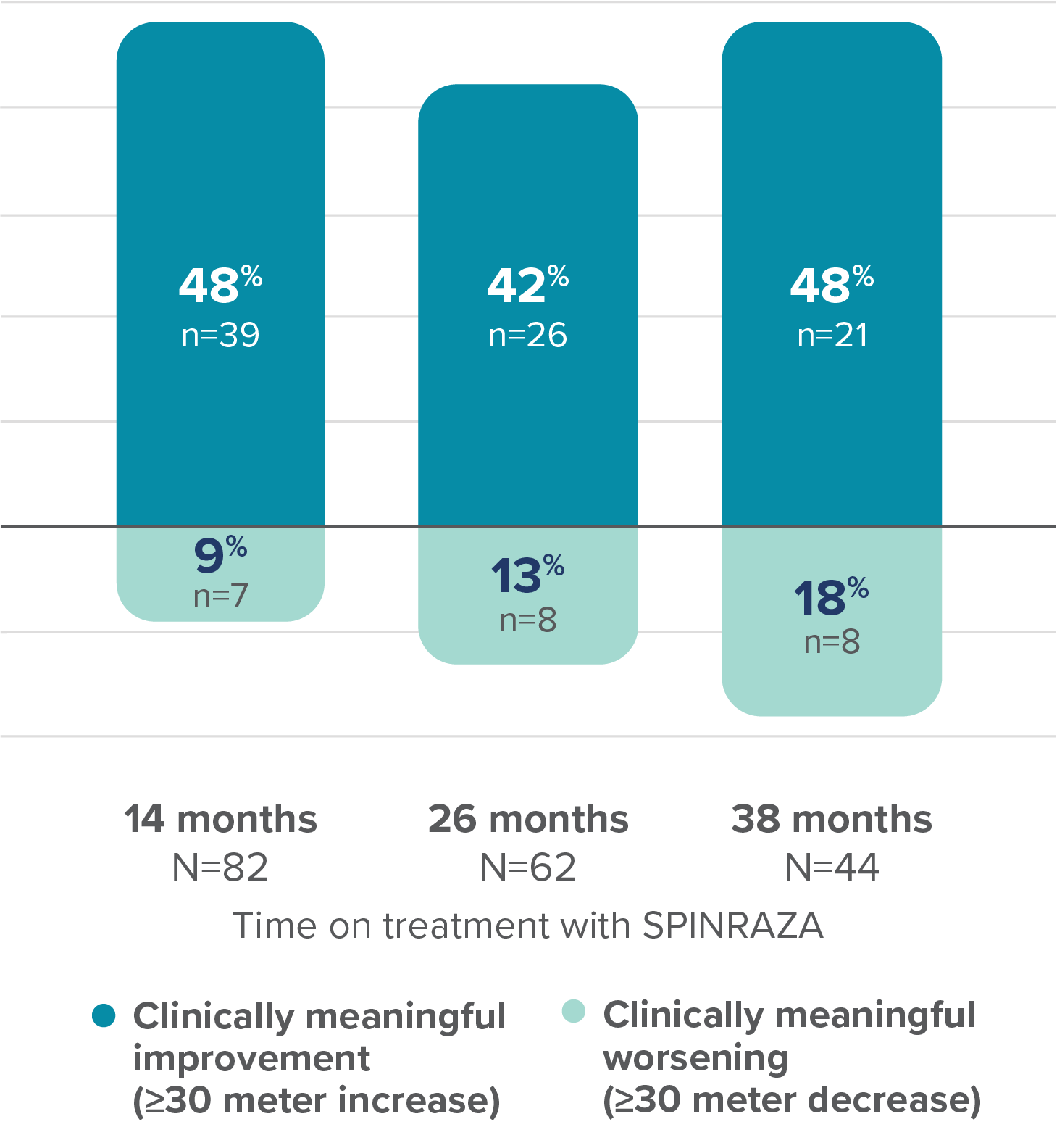
At 38 months, the mean improvement in walking distance in the 6MWT from baseline was 32.20 meters3
6MWT, 6-minute walk test; Cl, confidence interval; SD, standard deviation.
Exploratory subgroup analysis in ambulatory and non-ambulatory patients3‡
Mean improvement in HFMSE and RULM score from baseline


Regardless of ambulatory status, improvement in mean HFMSE and RULM scores were reported with SPINRAZA3
- HFMSE score interpretation in non-ambulatory patients may have been limited by floor effects; non-ambulatory patients could not perform some of the activities assessed by HFMSE, leading to lower achievable scores. RULM score interpretation in ambulatory patients may have been limited by ceiling effects, which may have affected the results of the study
‡Additional subgroups were reported in the study.
CI, confidence interval; HFMSE, Hammersmith Functional Motor Scale–Expanded; RULM, Revised Upper Limb Module; SD, standard deviation; SMA, spinal muscular atrophy.
Long-term outcomes with SPINRAZA: This real-world evidence informs on the safety and efficacy of long-term SPINRAZA treatment for up to 38 months in adults and teens with SMA3
Review the warnings and precautions, including thrombocytopenia, coagulation abnormalities, and renal toxicity1
Łusakowska et al Orphanet
Journal of Rare Diseases
This real-world evidence study in older children and adults on SPINRAZA evaluated functional assessments and patient-reported outcomes for up to 30 months2
Study design: Prospective, observational study that assessed 130 patients who were treated with SPINRAZA (12 mg) between March 2019 and January 2022 with SMA (Types 1c-3), at 2 centers in Poland. Final analysis included 120 treatment-naïve patients. Seven patients were excluded due to insufficient follow-up, and 3 discontinued, which includes a fatality that was considered unrelated to treatment.2
Study limitations2:
- Open-label, multicenter study in Poland without a control group
- Treatment practices may vary by country
- Number of patients with type 1c and type 2 were limited
- Data for later time points were limited, as many patients had not yet reached those time points
- Missing assessments on some endpoints (eg, 6MWT) due to COVID-19 restrictions
- CHOP INTEND is not validated in adults
- Differences in dosing compared to approved SPINRAZA dosing schedule
- PGI-I is subjective, patient-reported, and not validated in SMA
Endpoints2:
- Mean change in HFMSE vs baseline up to 30 months
- Mean change in CHOP INTEND score between month 0 and month 26
- Mean change in RULM score vs baseline up to 30 months
- Mean change in 6MWT score vs baseline up to 30 months
- PGI-I assessment at subsequent time points of treatment
Safety: The study safety profile is generally consistent with the safety reported in the SPINRAZA clinical trials1,2
- The most frequent adverse event was post-lumbar puncture syndrome (PLPS), reported in 198 patients (19%)2
- All patients with PLPS reported headache, mainly of mild intensity2
- Cerebrospinal fluid leak was observed after CT-guided injection at month 10 for 1 patient2
- The leak stopped within 1 hour without intervention2
In this real-world study, there were 1,023 intrathecal administrations and no administration failures2
6MWT, 6-minute walk test; CHOP INTEND, Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders; COVID-19, coronavirus disease 2019; CT, computed tomography; HFMSE, Hammersmith Functional Motor Scale—Expanded; PGI-I, Patient Global Impression-Improvement; PLPS, post-lumbar puncture syndrome; RULM, revised upper limb module; SMA, spinal muscular atrophy.
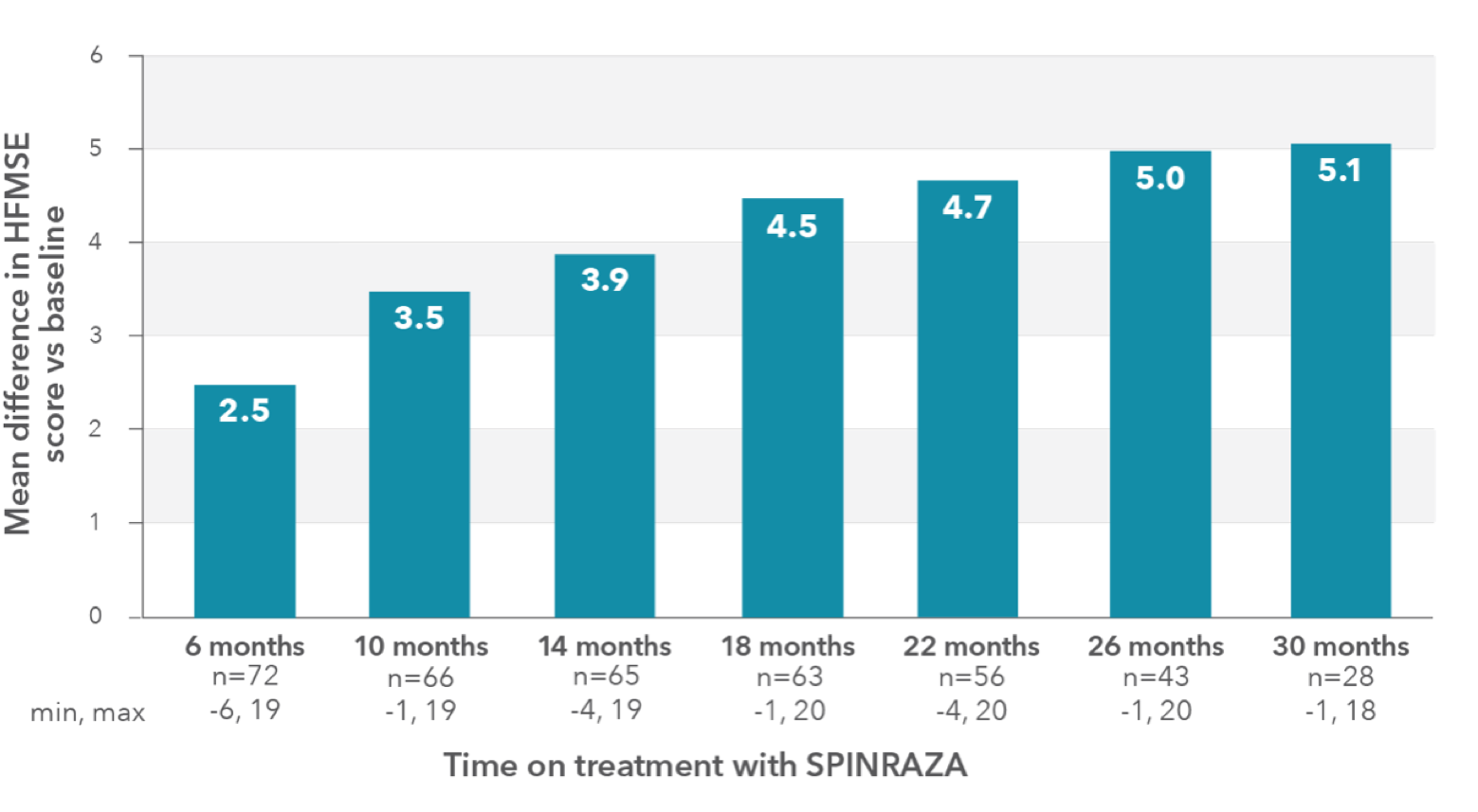
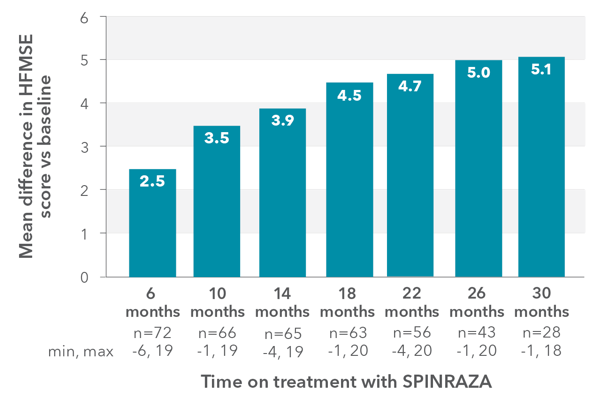
In the observational study, mean HFMSE score improved compared with baseline at each time point2
HFMSE patient population (n=73*):
- Mean age: 31 years (SD, 15.6 years; range, 5–66 years)
- Mean disease duration: 23.7 years (SD, 14 years; range, 4–62 years)
- SMA Type 2: 6 patients (4 children)
- SMA Type 3: 67 patients (10 children)
RESULTS:
- Mean HFMSE score increase of 5.1 points at month 30 (n=28)
Mean change in HFMSE vs baseline up to 30 months
In the study, a ≥3-point improvement was considered a clinically meaningful change
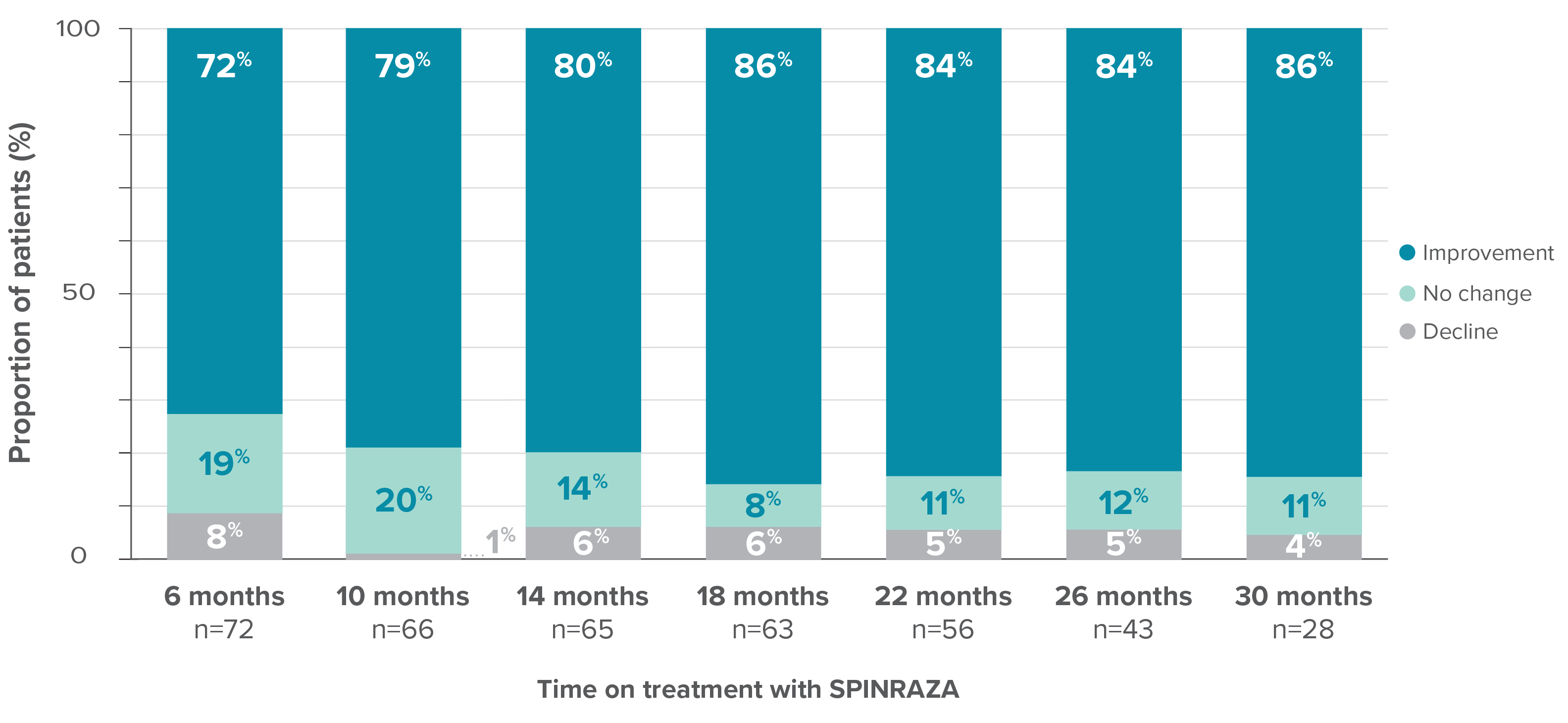
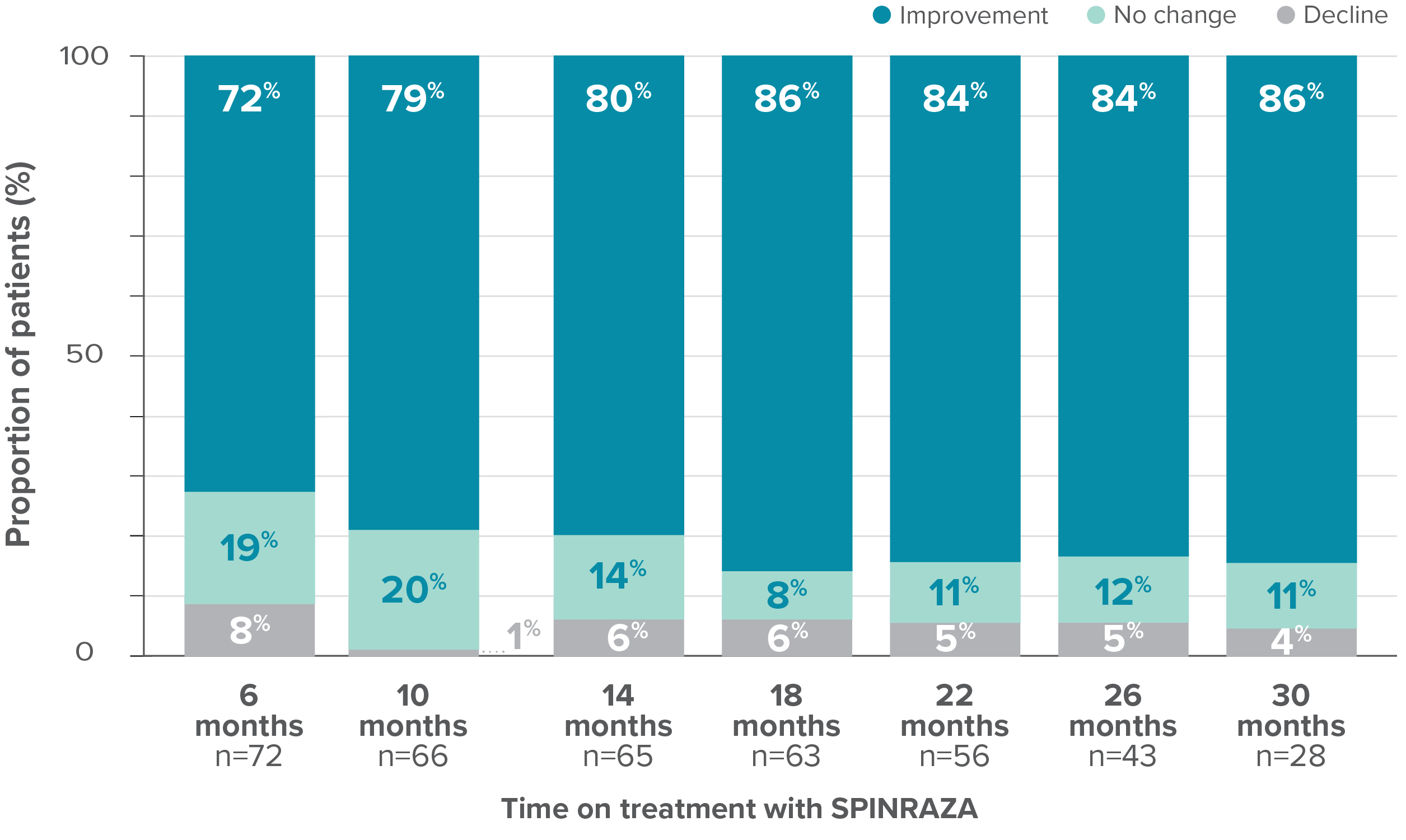
Proportion of patients who showed improvement, no change, or decline in HFMSE score vs baseline up to 30 months
- In 15% of patients (11/73), the HFMSE score improved by ≥10 points during the study
*One patient with SMA type 2 did not undergo assessment at day 180 (month 6) but was assessed at the subsequent 4 time points. Therefore, he was included in the analysis. At month 30, 28 patients were evaluated using the HFMSE.
HFMSE, Hammersmith Functional Motor Scale—Expanded; SD, standard deviation; SMA, spinal muscular atrophy.
At month 30, the HFMSE score showed improvement or no change in 96% of patients (27/28) on treatment with SPINRAZA2
Mean CHOP INTEND score improved compared with baseline at each time point2
CHOP INTEND patient population (n=47† ):
- Mean age: 33.7 years (SD, 11.0; range, 13-66 years)
- Mean disease duration: 31.7 years (SD, 9.5; range, 3.0-58.0 years)
- SMA type 1: 12 patients‡
- SMA type 2: 13 patients
- SMA type 3: 22 patients
RESULTS:
- Mean CHOP INTEND score increased by 5.6 points at month 26 vs baseline
- Clinically meaningful improvements (≥4 points) in the CHOP INTEND score were seen in 20.5% of patients (9/44) at month 6 and in 65% of patients (11/17) at month 26
Mean change in CHOP INTEND score between month 0 and month 26§
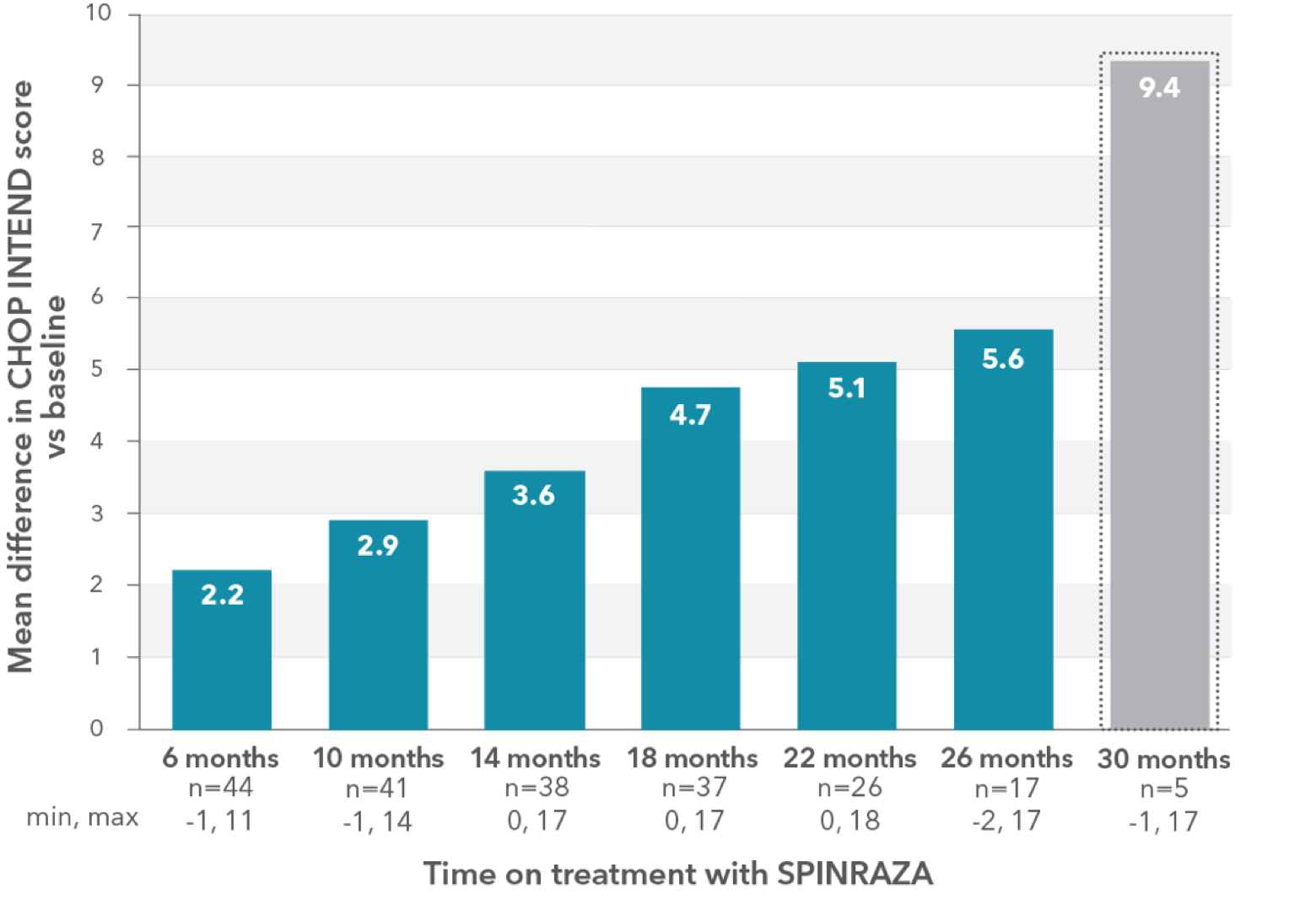
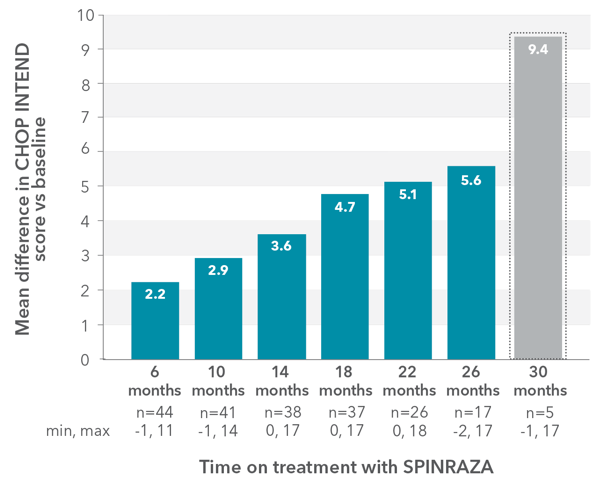
- 5 patients were available for assessment at month 30; the mean difference was 9.4 points
The CHOP-ATTEND test validated for adult patients with severe symptoms was not available at the time of the study. For this reason, the CHOP INTEND test, which is not validated in adults, was used.
† Forty-four patients were assessed at least twice (at month 0 and month 6).
‡Baseline CHOP INTEND score was not available for 3 adults with SMA Type 1 who started treatment abroad within the Expanded Access Program (EAP). They started study evaluation at month 10, month 14, and month 18, respectively. Assessment was available up to month 30 for 2 of these patients.
§Low patient numbers (n=5) at 30 months preclude meaningful interpretation at this time point.
CHOP-ATTEND, Children’s Hospital of Philadelphia Adult Test of Neuromuscular Disorders; CHOP INTEND, Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders; EAP, Expanded Access Program; SD, standard deviation; SMA, spinal muscular atrophy.
94% of patients (16/17) noted improvement by at least 1 point at month 26 vs baseline2
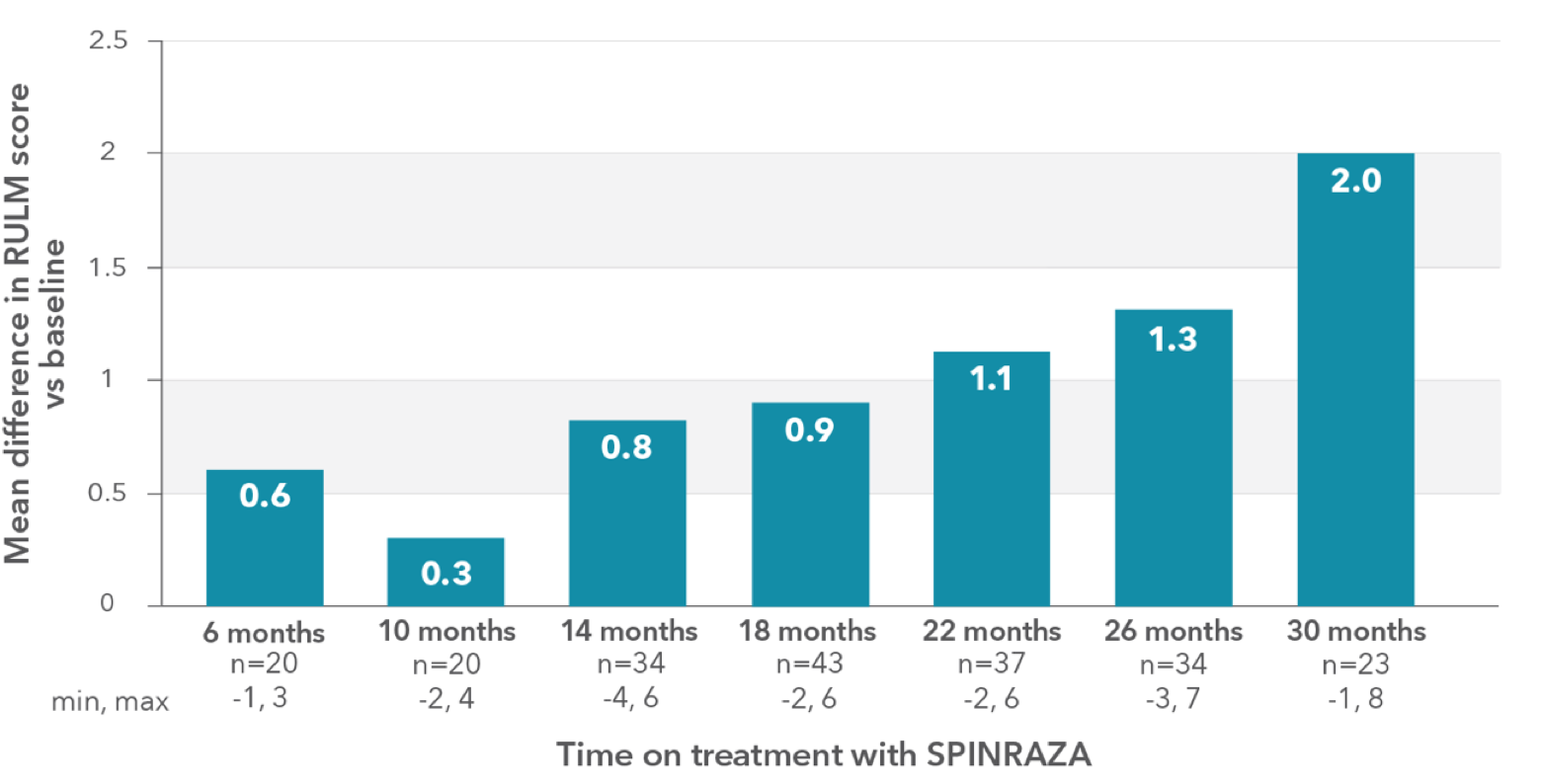
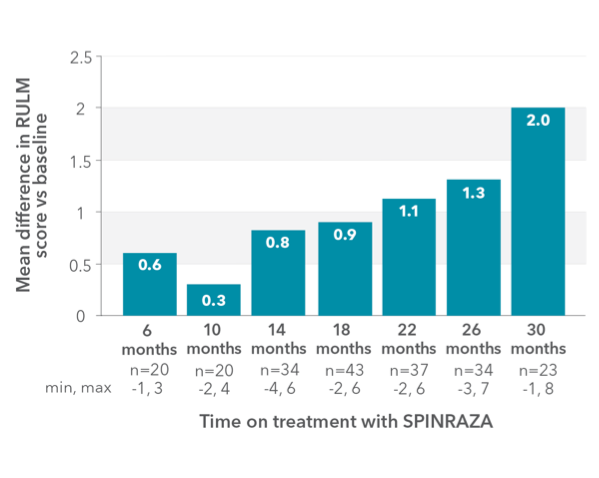
RULM score improved compared with baseline at each time point2
RULM patient population (n=51):
- Mean age: 27 years (SD, 14; range, 5-66 years)
- Mean disease duration: 22 years (SD, 12.8; range, 3.8-62.0 years)
- SMA type 2: 9 patients
- SMA type 3: 43 patients
RESULTS:
- Mean RULM score increased by 1.96 points at month 30 vs baseline
Mean change in RULM score vs baseline up to 30 months
Proportion of patients who showed improvement, no change, or decline in RULM vs baseline up to 30 months
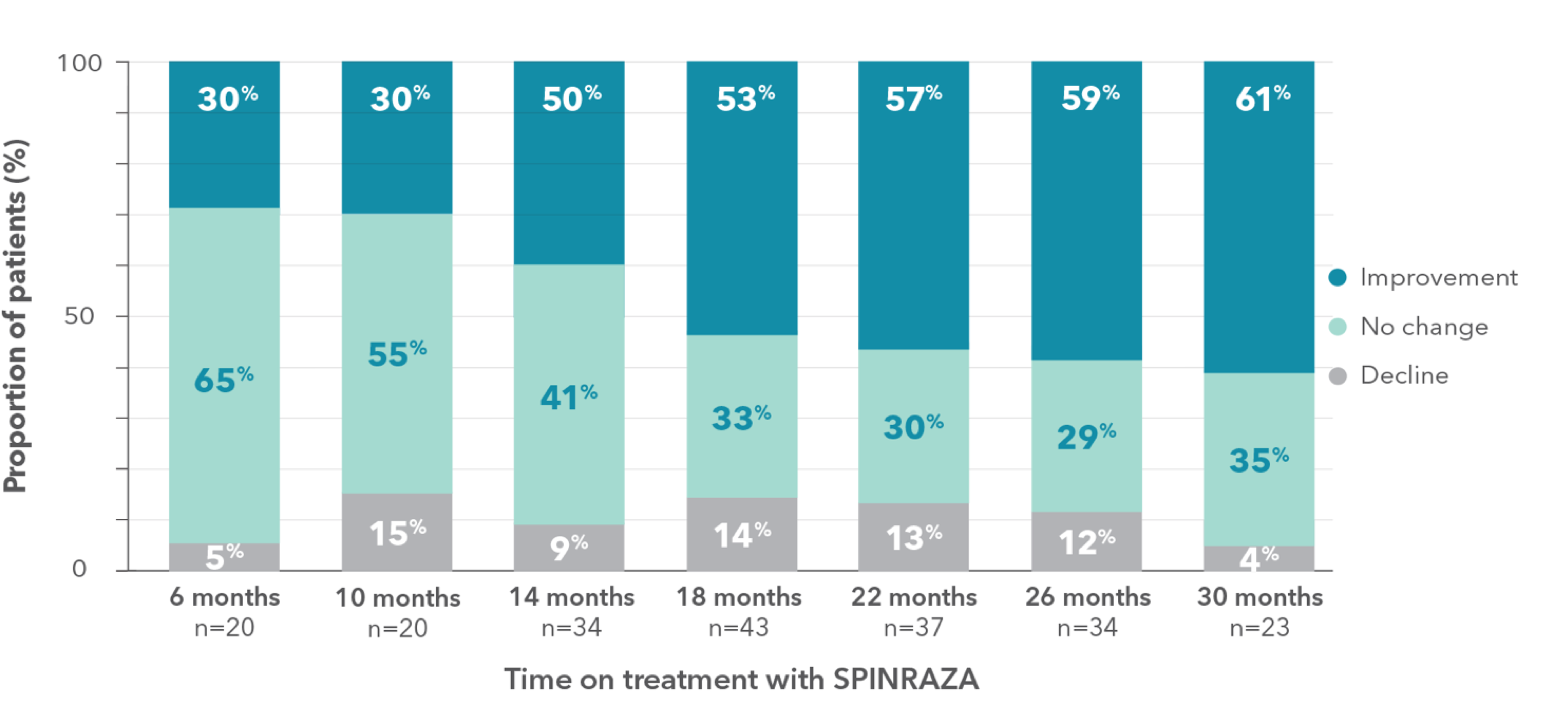
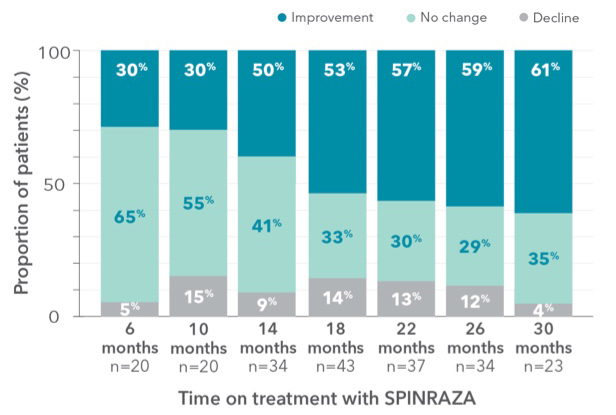
- During treatment, the proportion of patients who showed improvement in upper limb function was 61% (14/23) at month 30
RULM, Revised Upper Limb Module; SD, standard deviation; SMA, spinal muscular atrophy.
Clinically meaningful improvements (≥2 points) in RULM score was seen in 43.5% (10/23) of patients at month 302
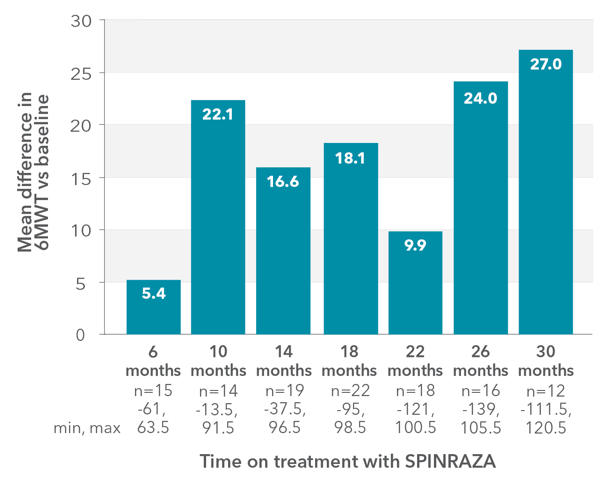
Mean 6MWT scores improved at each time point2
6MWT patient population (n=27 ||):
- Mean age: 27 years (SD, 13; range, 6-59 years)
- Mean disease duration: 18 years (SD, 10; range, 4-33 years)
- SMA type 3: 27 patients
RESULTS:
- Mean 6MWT improved by 27.0 meters at month 30 vs baseline
Mean change in 6MWT vs baseline up to 30 months

Proportion of patients who showed improvement, no change, or decline in 6MWT score vs baseline up to 30 months
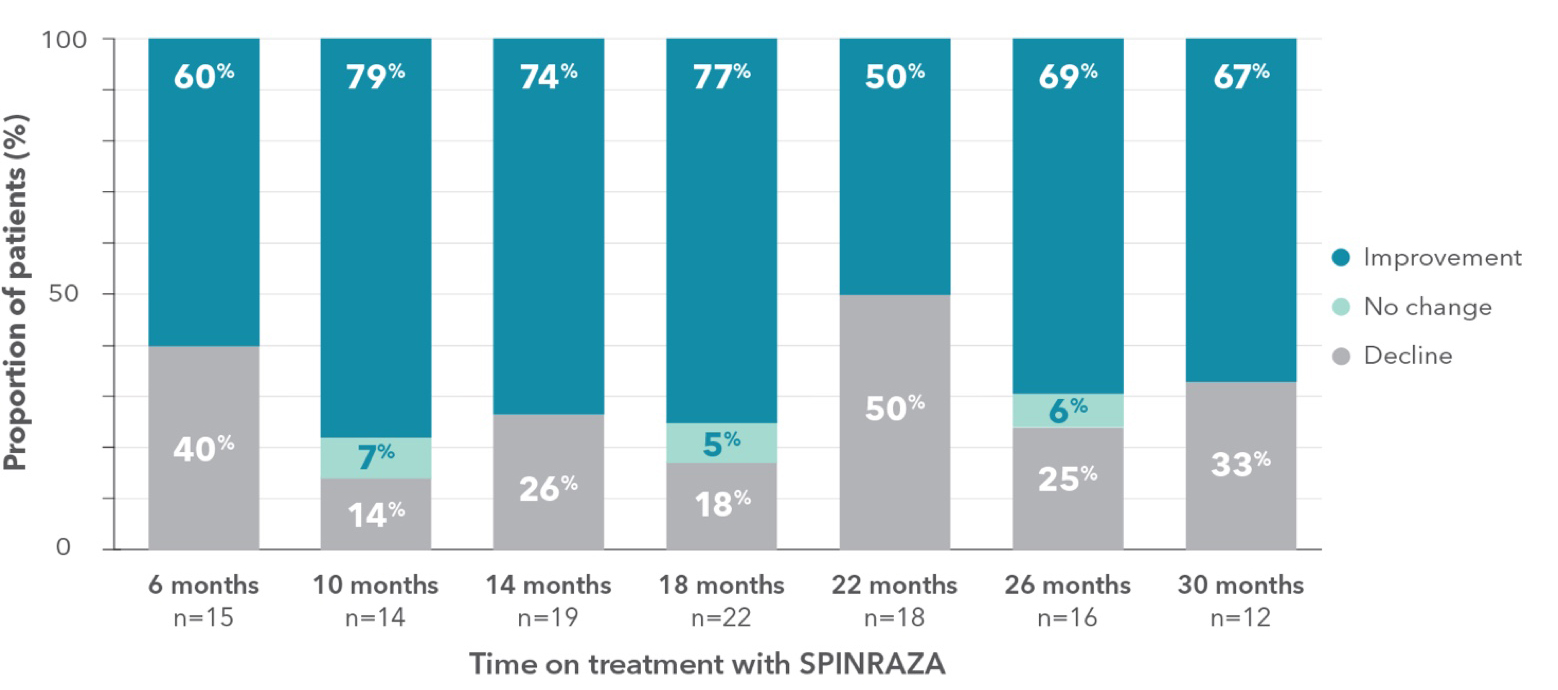
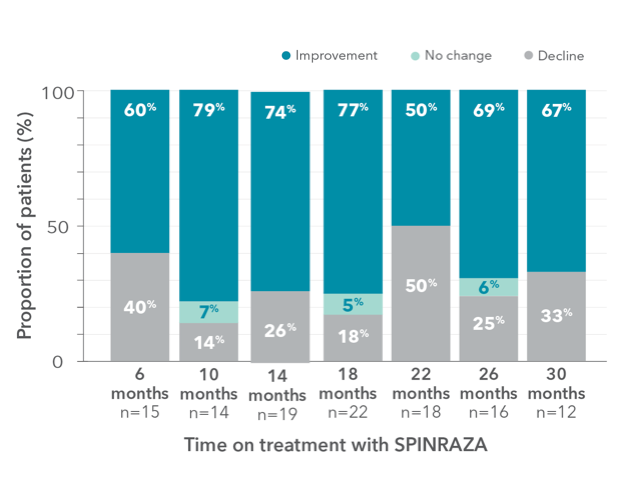
- A relatively large number of patients experienced worsening in each point of treatment: 40% of patients (6/15) at month 6 and 33% of patients (4/12) at month 30
||The lack of a fairly significant number of ratings in the 6MWT was mainly due to patients’ fear of staying too long in the hospital and contacting medical staff and other patients during the pandemic.
6MWT, 6-minute walk test; SD, standard deviation; SMA, spinal muscular atrophy.
Clinically meaningful improvement (≥30 meters increase in walking distance) was observed in 33% of patients (5/15) at month 6 and was 50% (6/12) at month 302
Improvements were seen across all functional assessments up to 30 months2
Distribution of patients who achieved a clinically meaningful improvement in each of the functional tests
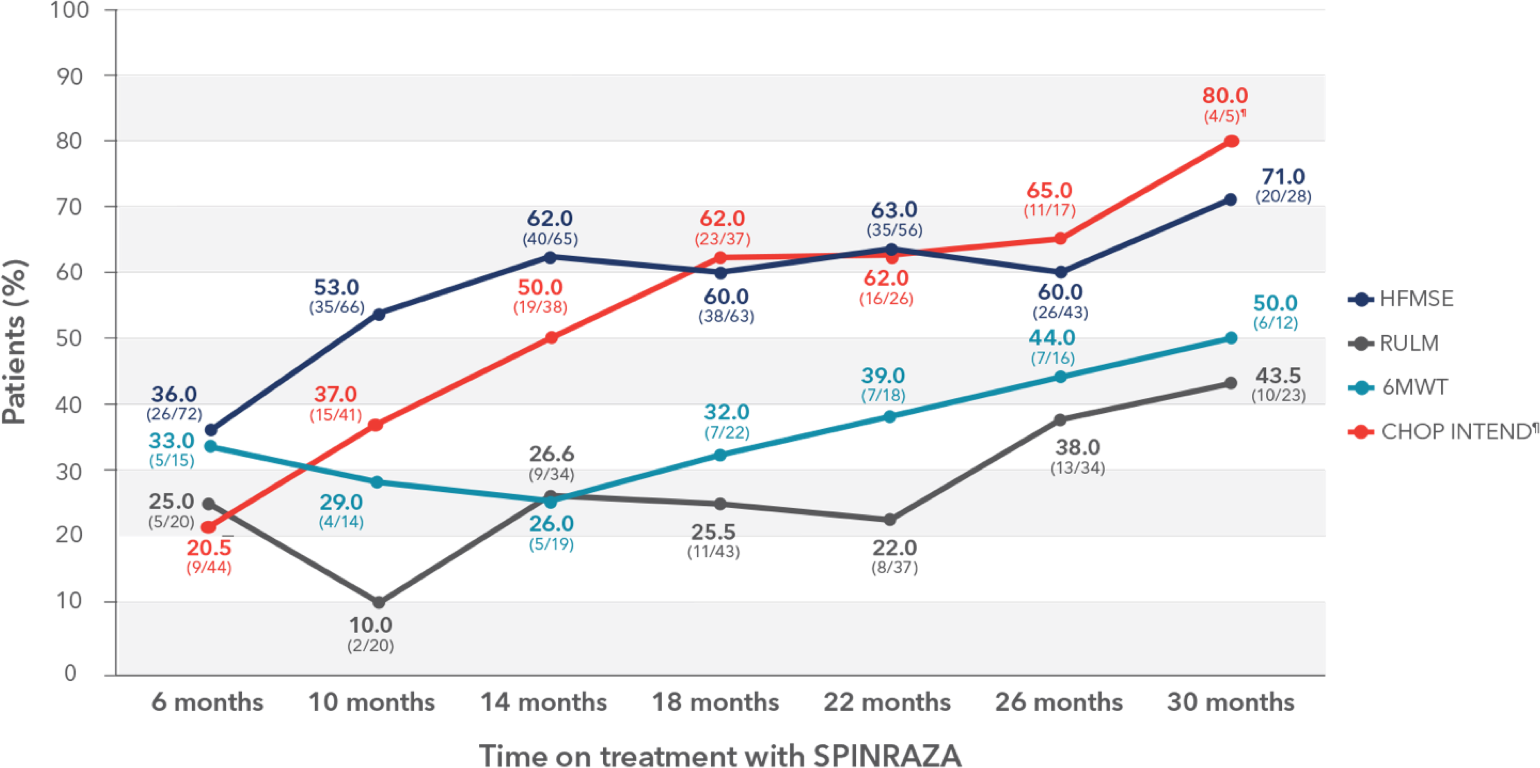

- Clinically meaningful improvement was defined as a change in HFMSE score of ≥3 points, change in CHOP INTEND score of ≥4 points, and change in RULM score of ≥2 points. For patients able to walk independently, significant improvement in the 6MWT score was defined as an increase in walking distance by at least 30 meters
¶Three patients who did not undergo assessment at month 0 were excluded. Only 5 patients were evaluated for CHOP INTEND at month 30.
Patients and caregivers assessed and self-reported their status, and most reported improvement or stabilization while on treatment2#
- While no patients reported feeling much worse or very much worse at each time point, between 2 and 5 patients reported feeling minimally worse at various time points during the study
#PGI-I data was subjective and patient-reported.
6MWT, 6-minute walk test; CHOP INTEND, Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders; HFMSE, Hammersmith Functional Motor Scale—Expanded; PGI-I, Patient Global Impression–Improvement; RULM, Revised Upper Limb module; SD, standard deviation.
After 26 months of SPINRAZA treatment, 96.5% of patients (62/64) reported subjective improvement or stabilization/no change, which was also reported at month 30 in 100% (47/47) of the patients2
Review the warnings and precautions, including thrombocytopenia, coagulation abnormalities, and renal toxicity1
Hagenacker et al The Lancet Neurology
An independent, real-world study of teens and adults with up to 14 months of SPINRAZA5
Study design: An independent, prospective, multicenter, observational cohort study
Study duration: Up to 14 months. SPINRAZA (12 mg); Assessments made at 6, 10, and 14 months
Participants: 139 patients with genetically confirmed 5q later-onset SMA, aged 16 to 65
Primary endpoint: Change from baseline in motor function measured by HFMSE at 6, 10, and 14 months. Patients with missing baseline HFMSE scores were excluded from these analyses
Secondary endpoints: Change from baseline in upper limb and walking ability measured by RULM and 6MWT at 6, 10, and 14 months
Study limitations: No control group; observational design. Study powered on primary endpoint only
Safety: The majority of adverse events (AEs) were generally consistent with those reported in the SPINRAZA clinical trials. Other reported in the SPINRAZA clinical trials. Other reported AEs were:
- Nausea
- Diffuse pain
- Constipation
- Infection
- Meningitis, aseptic
- Vertigo
- Tinnitus, aggravated
- Bladder disorder not otherwise specified
6MWT, 6-minute walk test; AE, adverse event; HFMSE, Hammersmith Functional Motor Scale—Expanded; RULM, Revised Upper Limb Module; SMA, spinal muscular atrophy.
Not a Biogen-sponsored study. Biogen-sponsored pivotal trials for SPINRAZA did not include adults with SMA.
One of the largest real-world studies of SPINRAZA included 139 adults with later-onset SMA up to age 655,6
Primary endpoint: Mean change from baseline in HFMSE score (95% CI)*
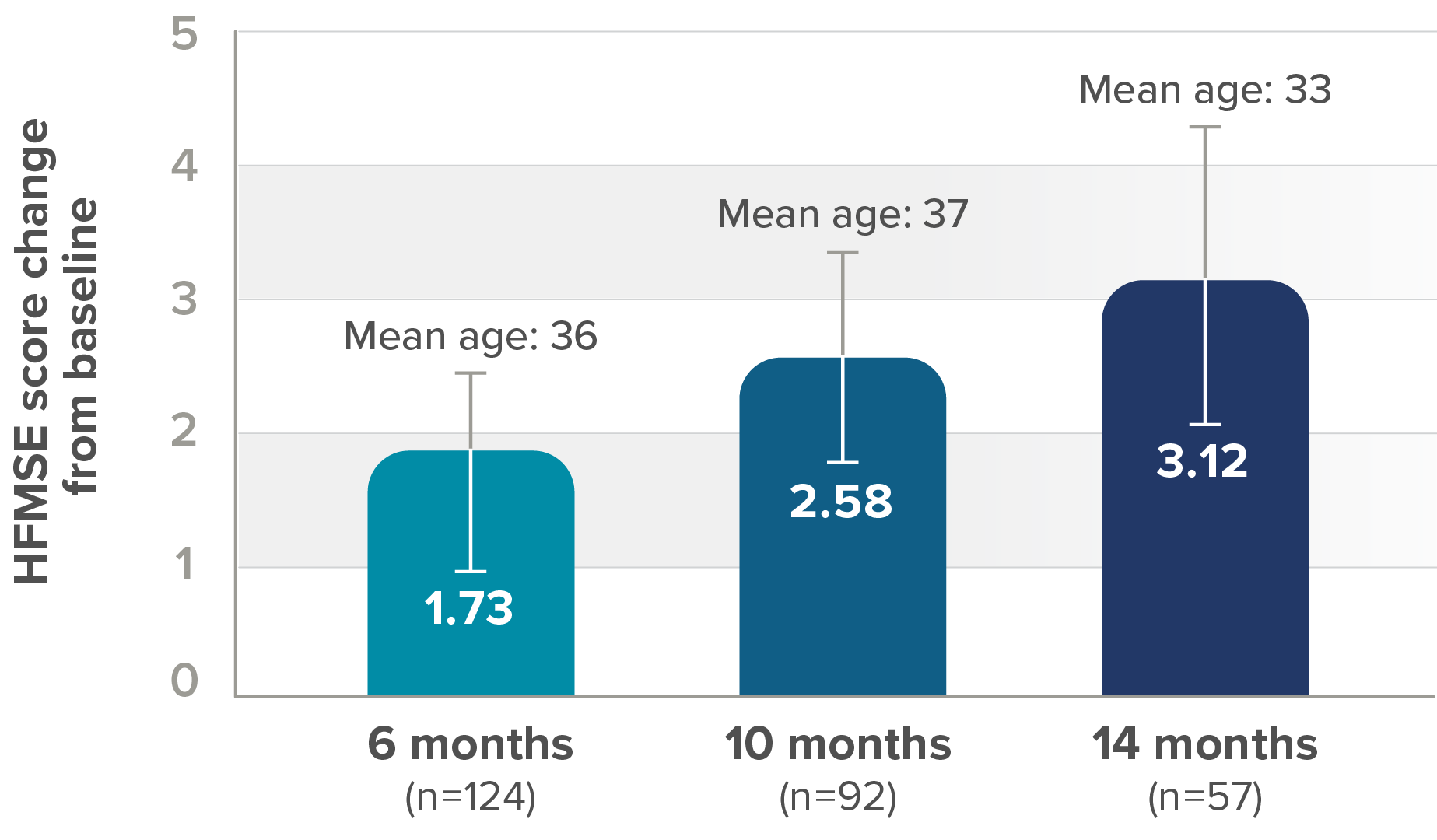
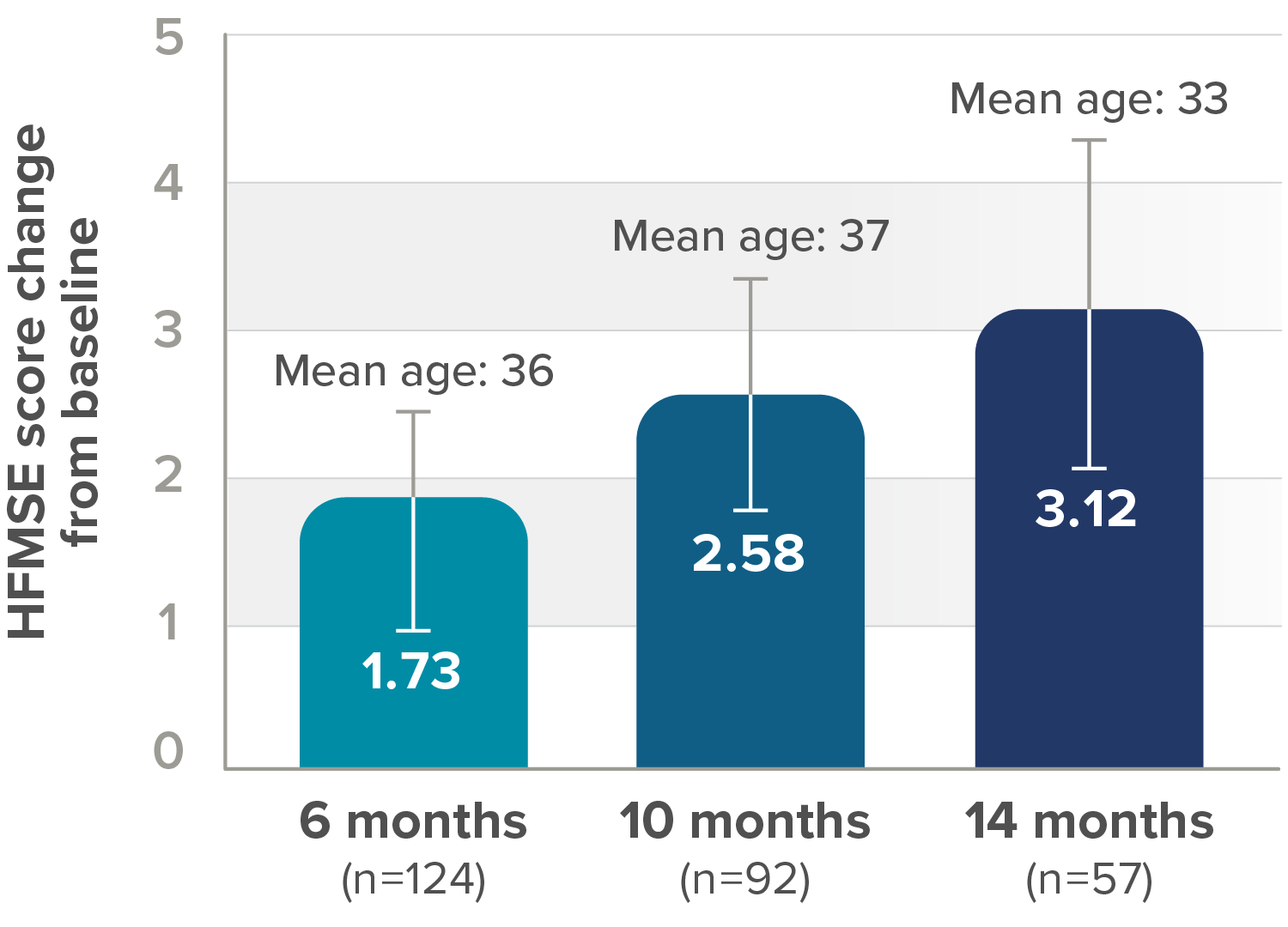
SPINRAZA increased mean HFMSE scores compared with baseline
Fourteen of 124 patients (11%) showed worsening motor function under treatment as measured by HFMSE. 139 patients completed an assessment at 6 months, 105 at 10 months, and 61 at 14 months. Patients not included at 10-month and 14-month assessments were those who had not reached the assessment time point (30 at month 10, 44 at month 14), were missing baseline or assessment values (15, 13, and 4 at month 6, 10, and 14, respectively), had an adverse reaction or procedure-related event (n=2), or withdrew consent (n=2). Greater improvement of motor function was correlated with lower severity of disease at baseline.
*Lower bound 95% CI not shown.

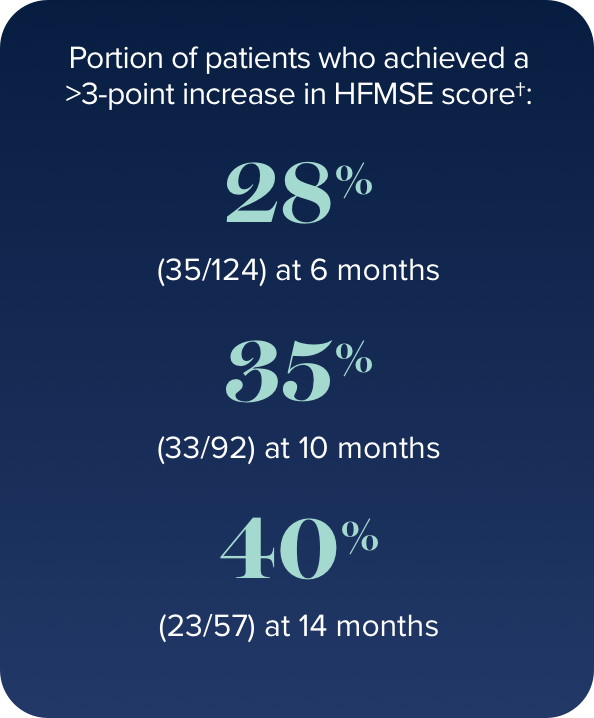
†Exploratory endpoint.
A ≥3-point increase is considered clinically meaningful for HFMSE. A 1-2–point increase could be considered a positive outcome in a disease where natural history has shown a progressive decline.5,7
CI, confidence interval; HFMSE, Hammersmith Functional Motor Scale—Expanded; SMA, spinal muscular atrophy.
SPINRAZA improved mean upper limb function and walking distance compared with baseline at every study time point5
Secondary endpoint: Mean change from baseline in RULM score (95% CI)‡
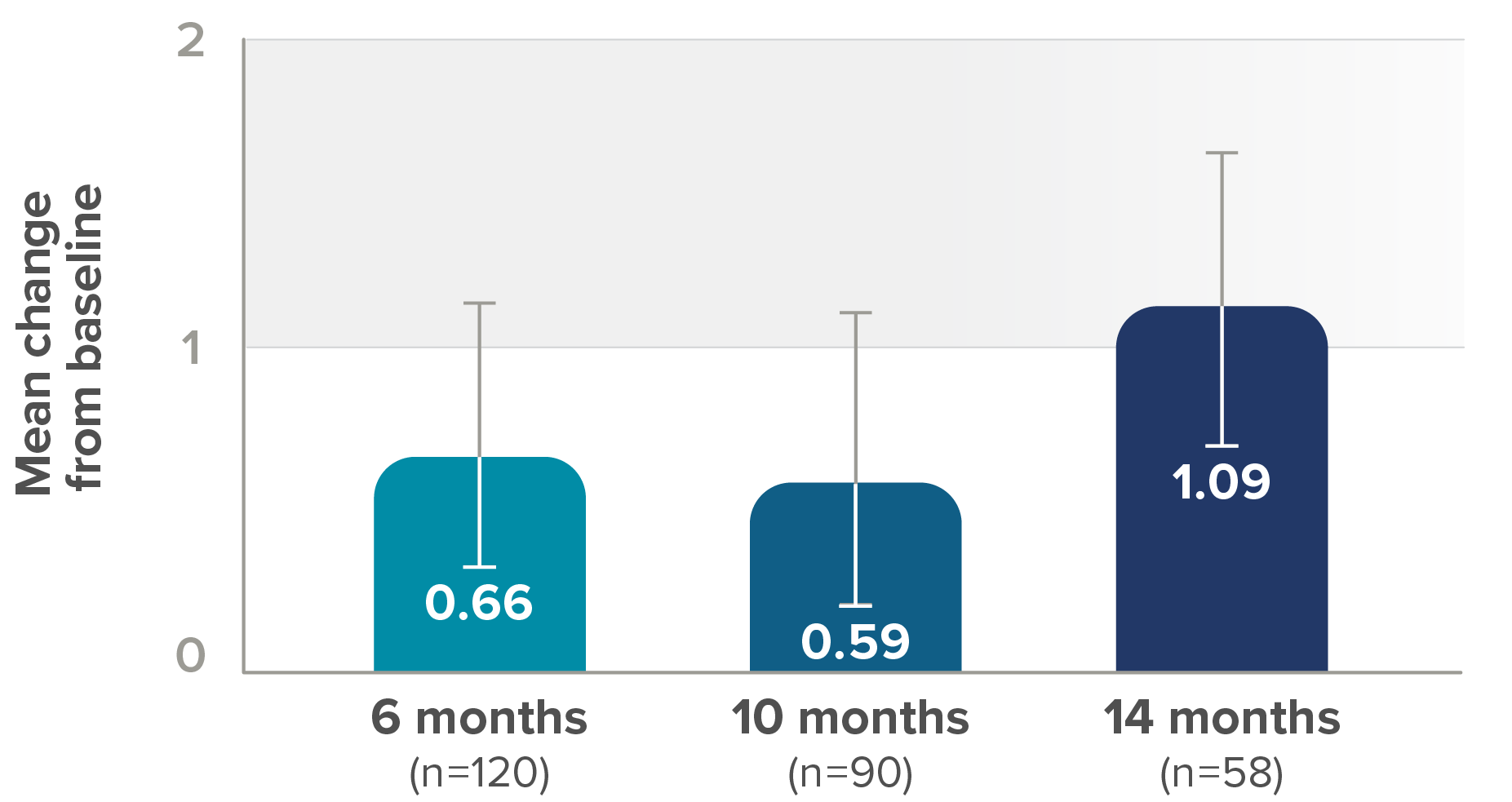
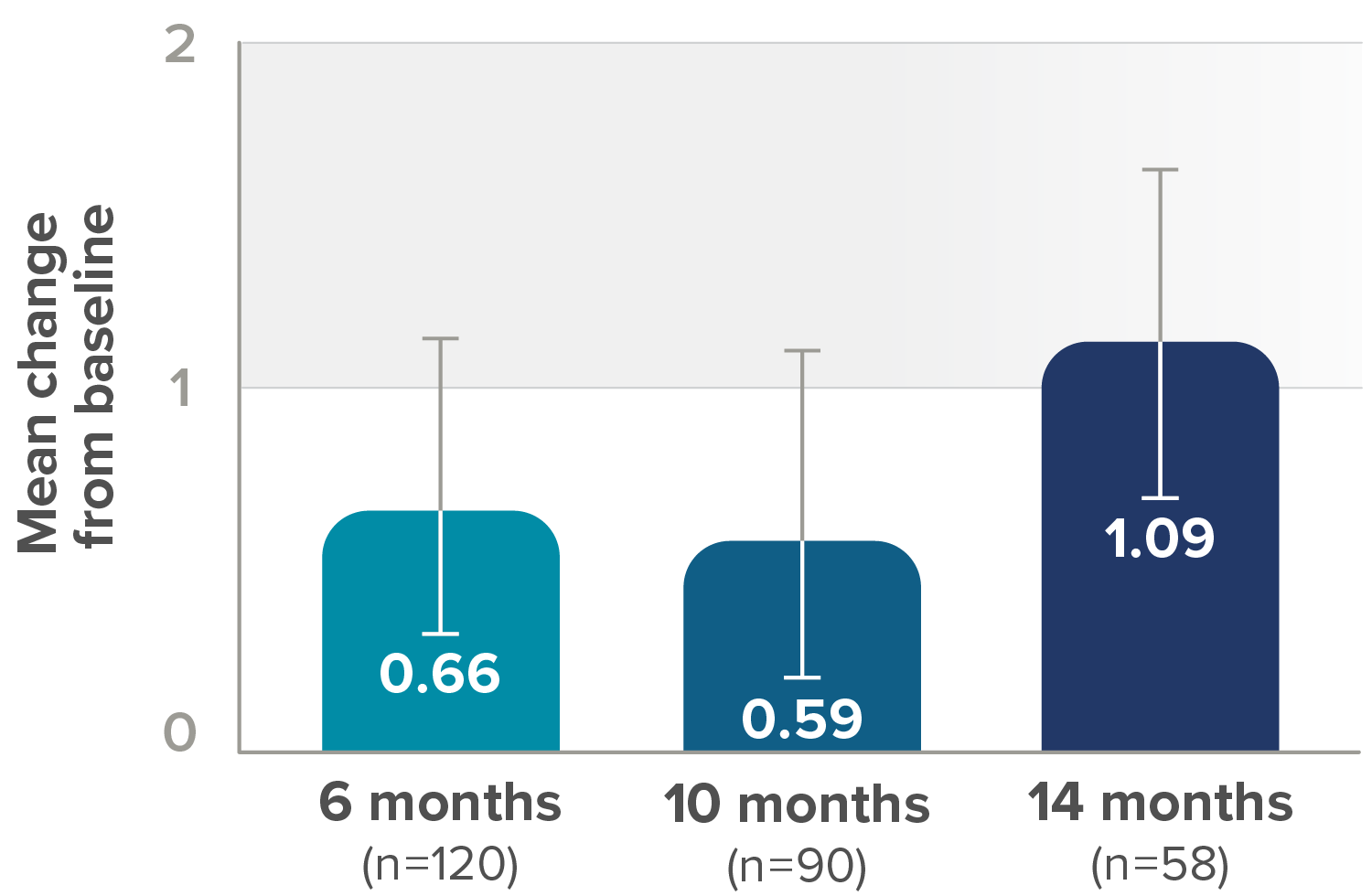
- 75% (21/28) who saw clinically meaningful improvements§ in RULM score at 6 months maintained these milestones at 14 months
- At 6 months, 28 (23%) of 120 patients showed ≥2-point improvement in RULM score from baseline (ie, a clinically meaningful improvement), whereas 74 (61%) showed no meaningful change, 18 (15%) showed a decline of 1 point or more, and 10 (8%) showed a decline of ≥2 points
‡Lower bound of 95% CI not shown.
§For individuals with later-onset SMA, clinically meaningful was defined as an improvement in RULM score of at least 2 points.
CI, confidence interval; RULM, Revised Upper Limb Module.
Secondary endpoint: Mean change from baseline in 6MWT score (95% CI)||
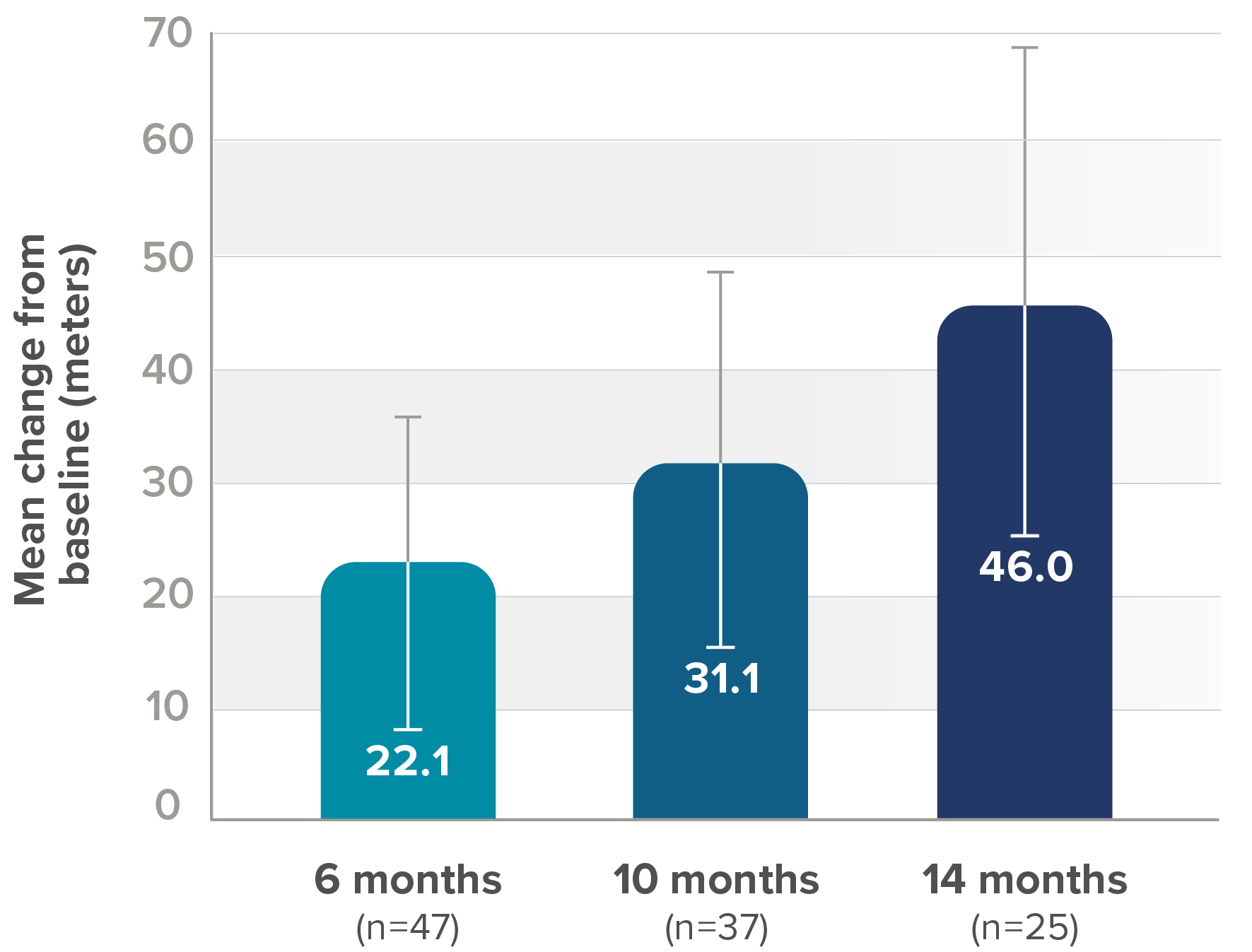
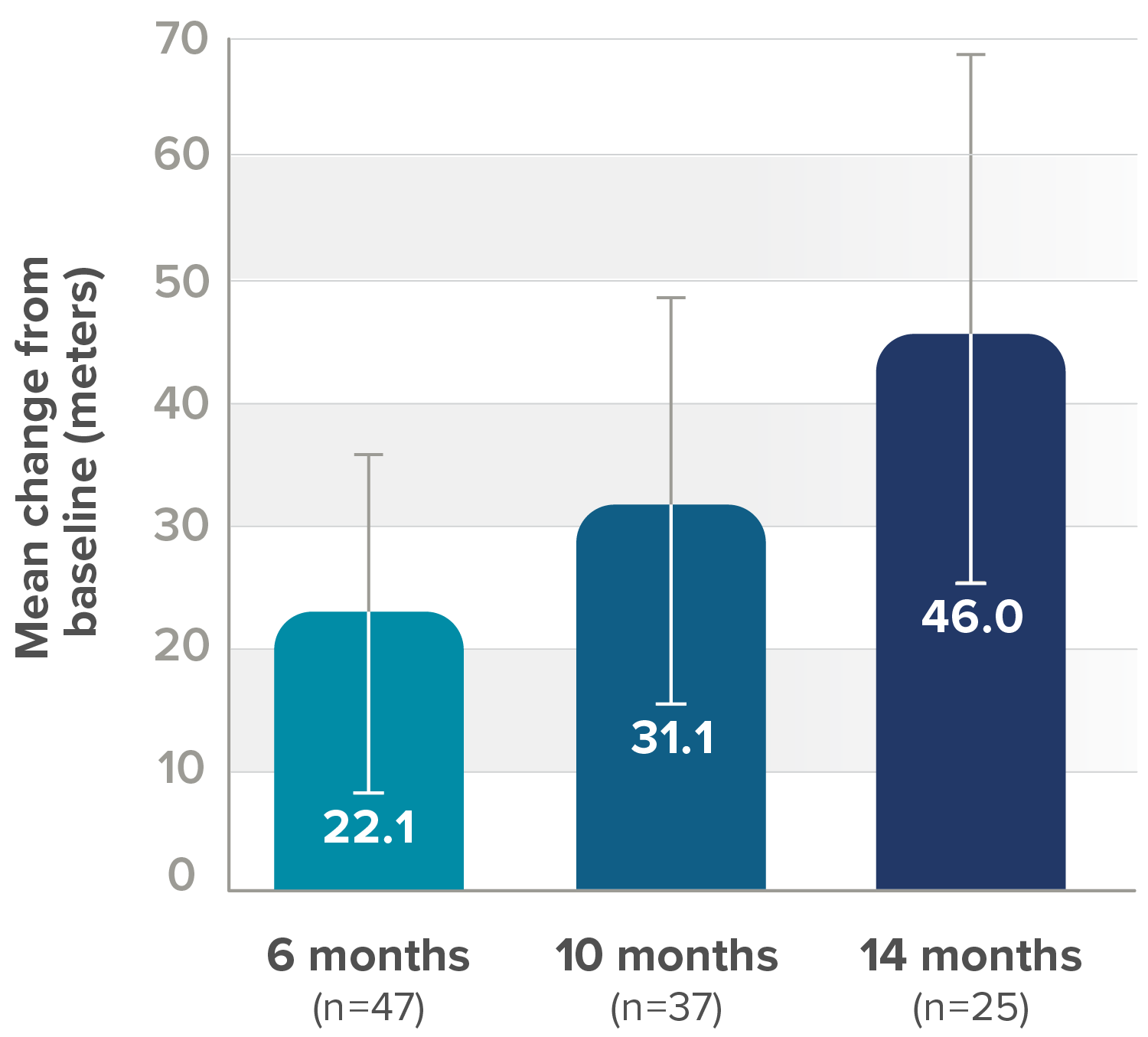


||Lower bound of 95% CI not shown.
6MWT, 6-minute walk test; CI, confidence interval.
